Abstract
It is known that IL-10 plays a critical role in the resolution of inflammation or tissue damage and is the most widely studied anti-inflammatory cytokine, as discussed in different reviews. Since its initial discovery, IL-10 production has been observed in an array of leukocytic cell types and some non-immune cells. Considering recent findings, this review discusses the role of IL-10 in different pathological contexts. In this respect, IL-10 may be considered a manipulative tool that suppresses the much more effective T helper 1 profile which is produced upon the influence of infective agents. The increased IL-10 concentration, which persists for a period of days to a few weeks, is associated with influencing various diseases’ outcomes, and its implications are observed in different tissues and processes, including infections, traumas, regeneration, or hyperthermia during physical activity. These findings reinforce the concept that IL-10 should be used in association with co-stimulatory effectors as necessary to exert the appropriate influence during the management of inflammatory or infective pathologies. Hopefully, further findings can open new avenues to study the biology of this cytokine and its therapeutic potential.
INTRODUCTION
IL-10 plays an important role in the attenuation of inflammation or tissue damage and has been found to be produced by an array of white blood cell types, including lymphocytes, monocytes, and granulocytes, as well as non-immune cells such as epithelial or neuronal cells.1-4 IL-10 acts through a trans-membrane receptor complex, which is composed of IL-10R1 and IL-10R2, and regulates the functions of lymphocytes, macrophages, and various other cells.5,6 Several infection studies support the idea that IL-10-producing cells, including T regulatory cells (Treg), macrophages, and dendritic cells (DC), are a major subset of immune cells, possessing potent suppressive properties directed at T effector cells.1 Additional IL-10-producing cells are polymorphonuclear cells, natural killer cells (NK), and B regulatory cells (Breg), which are involved in infective, autoimmune, and neoplastic diseases, as well as tolerance induction.1,5,6
Inducible IL-10-secreting Breg have also been demonstrated to contribute to allergen tolerance through suppression of effector T cells and selective induction of IgG4 isotype antibodies.1,7,8 The allergen-tolerant state after exposure to high concentrations of pathogen-associated molecular patterns is associated with the local and systemic induction of distinct populations of allergen-specific T regulatory lymphocytes, including IL-10+ Treg, TGF-β+ Treg, and FoxP3+ memory Treg.9,10 The protective and recovery-promoting effects of IL-10 during autoimmune diseases (mainly produced by Breg or DC) include a reduction in peripheral T-cell proliferative responses via the modulation of antigen-presenting cell function, a decrease in pro-inflammatory cytokine secretion, and a preferential inhibition of T helper (Th)17-mediated neuro-inflammation; the defective expression of Breg combined with impaired Treg and enhanced Th17 cells play an important role in the development of autoimmune pathologies.1,11,12
Notably, several studies on infective diseases show IL-10 to be a crucial factor in inhibiting the harmful effects of the innate pro-inflammatory immune response in a Th1-dominated milieu only, but not if the balance is shifted towards a Th2 response.1,13-15 The induced manipulation of T cells and B cells toward the Th2 profile and macrophage/monocytes activation can lead to impaired resistance, therefore assuring chronicity and an increased survival potential for different infective agents, such as viruses, chlamydia, protozoans, and parasites.13,14,16-19
IL-10 production also induces extensive changes in gene expression and cytokine release, which also lead to endotoxin tolerance and deviations in cellular function, maintenance, growth, and proliferation, as well as coagulation and fibrinolysis, cell–cell signalling or interaction, and cellular movement.1,20,21 In this review, the authors discuss the role of IL-10 in different situations in light of recent findings.
INFECTIVE PATHOLOGIES, IL-10 TRANSIENT INCREASE, AND THE SWITCH OF IMMUNE RESPONSE
There have been numerous regulatory effects of IL-10 described, predominately related to infective pathology regulation (Table 1).
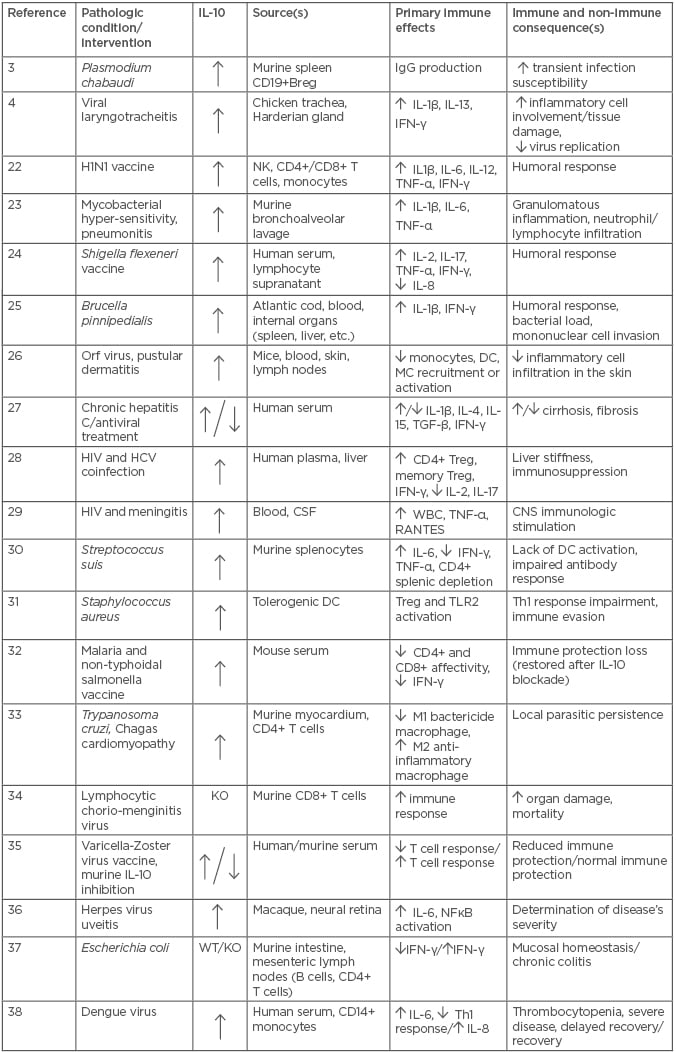
Table 1: The role of interleukin-10 in infective pathologies (2016–2018).
/: versus; Breg: B regulatory cells; CD: cluster of differentiation; DC: dendritic cells; HCV: hepatitis C virus; HIV: human immune-deficiency virus; H1N1: swine flu; KO: knock out; IFN: interferon; CNS: central venous system; CSF: cerebrospinal fluid; Ig: immunoglobulin; M: macrophage type; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; NK: natural killer; RANTES: regulated on activation, normal T expressed and secreted; TGF: transforming growth factor; Th: T helper; TLR: toll-like receptor; TNF: tumour necrosis factor; Treg: T regulatory cells; WBC: white blood cell; WT: wild type.
Accordingly, spleen analysis of the CD19+ Breg cell response on Plasmodium chabaudi-infected BALB/c mice reinforced the observation of its regulatory role during an infection-related phenotype shift, represented by a strong IL-10 production.3 Similarly to infections, vaccination with monovalent H1N1 influenza is associated with a broad spectrum upregulation of inflammatory and regulatory biomarkers, such as IL-1β, IL-6, IL-10, and IL-12 derived from NK, CD4+, and CD8+ T-cells.22 This immune response relies on antibody production to provide persistent immune protection against the influenza antigen that subjects are exposed to.
Previous findings regarding functions of the mentioned cells indicate that IL-10 is mainly produced during transient immune conditions and that the persistent IL-10-related effect may be the effectuation of the switching immunological response.1 These effects are demonstrated during exposure to infective agents, immunotherapy to different allergens, and the development of autoimmune pathologies, in which the implication of Th1, Treg, or Breg cells lead to the production of specific antibodies.1,3-21 Recent evidence appears to support IL-10 being a switcher of immune response.4,23-27 Thus, the peak of pro-inflammatory (IL-1β, IFN-γ) and anti-inflammatory (IL-10, IL-13) cytokine gene transcription, 5 days post-infection with an infectious laryngotracheitis virus strain in chickens, coincided with an increased recruitment of inflammatory cells, extensive tissue damage, and limiting of virus replication in the trachea.4 Early responses in an acute model of mycobacterial hypersensitivity pneumonitis in mice revealed a time and dose-dependent increase in a range of cytokines, including TNF-α, IL-1β, IL-6, and IL-10, followed by subsequent granulomatous inflammation.23 Inactivated whole-cell Shigella flexneri 2a-vaccine also induced a transient increase of different cytokines, including IL-2, IL-10, IL-17, IFN-γ, and TNF-α.24 Brucella pinnipedialis additionally stimulates IL-10 or IFN-γ production, as observed from the 1st—28th day of experimental challenge in Atlantic cod, whereas anti-Brucella antibodies were detected from Day 14 onwards.25 A transient increase of IL-10 production during skin orf virus infection in mice was associated with recruitment limitation and trafficking inhibition of certain white blood and connective tissue cell subpopulations.26 In contrast, hepatitis C virus (HCV)-infected subjects show IL-10 reduction to normal levels only after successful antiviral treatment.27 In concert, these observations suggest that the increased IL-10 concentration under the influence of infective agents persists during the period of immune switch or modulation.1 The post-infective balance stabilisation for immune response compounds could correspond with the restoration of the lower (normal) IL-10 concentration to pre-infection levels.
INFECTIVE AGENTS, IL-10, AND MANIPULATION OF IMMUNE RESPONSE OR DISEASE OUTCOMES
Infectious agents are believed to be manipulators of immune response due to implications for IL-10 production, as reported in several studies. In this respect, HIV/HCV-co-infected patients have shown an immunosuppressive profile compared to healthy controls and HIV-mono-infected patients.28 White blood cell and inflammatory responses in cerebral liquor during asymptomatic bacterial meningitis in HIV-positive subjects has suggested that the central nervous system immune response in patients with HIV infection was independent of the systemic immune response.29 The manipulation of immune-responsiveness is also observed during Streptococcus suis infection, showing a modulation of DC functions.30 S. suis mouse splenocytes produced different cytokines, such as IL-6 and IL-10, but the level of Th1 cytokines TNF-α and IFN-γ were very low. Altogether, these results suggest S. suis interferes with the adaptive immune response.30 Staphylococcus aureus uses highly efficient immune evasion strategies to cause immune tolerance and results in a wide range of pathologies; the central mechanism corresponds to DC-related production of high amounts of IL-10, which is associated with an impaired Th1 response.31 Similarly to bacterial agents, malaria-related IL-10-production inhibited protection against an attenuated non-typhoidal Salmonella vaccine in mice co-infected with malaria in a transient manner.32 The infection-related Th1 response impairment may be an adaptive mechanism by the infective agent, because the Th1 immune profile is much more effective against infections than the Th2 isoform.1
Recent clinical and experimental studies on infective diseases also report the outcome deviation caused by IL-10, such as the failure to eliminate Varicella-Zoster virus, the deviation of parasite load within the myocardium during the acute phase of Chagas cardiomyopathy, or immunopathology exacerbation in select organs, ranging from transient local swelling to an increased risk for mortality during acute primary infection with the lymphocytic choriomeningitis virus.33,34,39 Gershon et al.35 specified that significant humoral immunity boosting after zoster vaccine only occurred in patients with a low constitutive IL-10 levels, while Jacobshagen et al.34 highlighted the physiological role of IL-10 in the regulation of a balanced T-cell response, also limiting the immunopathological damage. Moreover, the modulation of immune response and disease outcome, under variations of IL-10 level in a murine model of infection by Trypanosoma cruzi, is associated with a sudden switch from the classical M1 macrophage (microbicidal) phenotype toward an alternative M2 (repairing/anti-inflammatory) phenotype that occurred within the myocardium very shortly after infection.33 Considering that parasite persistence within myocardium is a necessary and sufficient condition for the development of the chronic myocarditis, Ponce et al.33 discovered that transient inhibition of the aforementioned macrophage switch enhanced the microbicidal M1 subset predominance, diminished IL-4 and IL-10-producing CD4+ T cells, promoted a pro-inflammatory cytokine milieu, and reduced parasite load within the myocardium during the acute phase.
IL-10 involvement with disease outcomes during infective pathologies is also observed in herpes simplex-infected neuronal retina, and mesenteric lymph nodes infected with Escherichia coli.36,37 According to Wu et al.,37 production of IFN-γ rapidly and progressively declined after colonisation of wild-type but not IL-10-deficient mice. CD4+ and B cell-related IL-10 in wild-type mice peaked at Day 4 and subsequently declined, suggesting that E. coli may deviate the profile of the effector immune system in normal hosts for their own purposes, in parallel with induction of IL-10 that subsequently suppresses this response to mediate mucosal homeostasis. Additionally, severe dengue cases had low Th1 cytokines and a concurrent increase in inflammatory mediators such as IL-6, IL-8, and IL-10, which originate from CD14+ cells. The reduction in the levels of IL-8 and IL-10 were identified as the most significant markers of recovery from severe disease.38 Aside from demonstrating the cytokine’s manipulative abilities, these studies reinforce the finding that the immunoregulatory cytokine IL-10 can suppress Th1-cell immunity.34
The increased IL-10 production can be associated with deviations in infectious disease outcomes and may be influenced by the infective agents themselves.13 The suppression of the Th1 response (and development of other less effective anti-infection profiles), as well as the deviation of the disease’s outcome towards the less aggressive anti-inflammatory phenotypes may be a result of interactions between the host immunity and the infective agent.1 This could lead to lack of infection eradication (disease chronicity), because, apart from the point-of-view of host-related beneficiary purposes, the anti-inflammatory and a less aggressive response may allow the infective agent to persist longer or under more favourable conditions within the host (avoiding the early death for both organisms) to fulfil its life cycle. In this context, the increased level of IL-10 seems to be necessary to mediate the switch of immune response.1,13,40
IL-10, TRAUMA, PHYSICAL STRESS, AND REPAIRING PROCESSES
Recent publications report the IL-10 involvement in traumatic or physical stress situations, showing some similarities with pure infective conditions. Thus, the transient early implication of IL-10 in repairing processes after traumatic situations is also shown in the microglia of epileptic subjects, during the recruitment of macrophages accompanying the shortening of the early phases of skeletal muscle regeneration in mice, in the T cell response after stroke, or in response to intensive exercise in hot environments.41-44 In addition, Lentivirus-related IL10-production and the consequent reduction of the neuroinflammatory response among spinal cord-traumatised mice reduced neutrophil infiltration at both Day 7 and Day 28 of experimental trauma. Similarly to T. cruzi infection, this effect correlated with skewing of the macrophage population toward an anti-inflammatory M2 phenotype and improvement of motor function, suggesting reduced secondary damage and increased sparing.33,45 Bodaan et al.46 reported that delayed healing of equine limb wounds was characterised by intensified and extended pro-inflammatory signalling and an exacerbated innate immune response, concomitant with the absence of anti-inflammatory IL-10.46 Short-term treatment of these wounds with orf virus IL-10 has dampened inflammation and promoted repair processes without accelerating closure. Taken together, these results indicate that localised expression of anti-inflammatory factors, such as IL-10, can modulate the inflammatory response following various traumatic injuries, and may be a key component of a combinatorial approach that targets the multiple barriers to regeneration and functional recovery.45 However, any manipulation of the IL-10 response for treatment purposes should be considered very cautiously due to its potential hazards to the immune system.1
CONCLUSION
In light of recent findings, IL-10 (as a potential switcher of the immune response) may be a manipulative tool that, when produced due to the influence of infectious agents, suppresses the much more effective Th1 profile. Lasting transiently for a period of days or weeks, the presence of increased IL-10 is associated with deviations in disease outcomes, and its implications are observed in different tissues (e.g., muscles and nerves) and processes (e.g., infections, traumas, regeneration, or physical stress). These observations emphasise that IL-10 should be used in association with the necessary co-stimulatory immune effectors to determine the appropriate deviation during the treatment of respective pathologies.1 Hopefully, further findings can open new avenues to study the biology of this cytokine and its therapeutic potential.





