BACKGROUND
Myocarditis is a common precursor to dilated cardiomyopathy (DCM) and together they are the cause of approximately half of all heart transplants, constituting a major cause of sudden death in young adults.1 Frequently initiated by viral infection of cardiomyocytes, approximately one third of those with acute myocarditis develop a chronic autoimmune syndrome leading to DCM and heart failure (HF).2,3 The pathogenesis and treatment of the progressive disease is not well established; therefore, uncovering the molecular basis of why patients do not recover normal ejection fraction within a year following onset of the disease is needed to develop new biomarkers and therapies. Cardiac myosin released from the damaged heart is a damage-associated molecular pattern that binds to toll-like receptor 2 (TLR2)4,5 and TLR8,5 promoting a Th17 pathogenesis in chronic autoimmune DCM and inducing anti-heart autoimmunity.6 Previous studies have demonstrated elevated anti-heart and anti-human cardiac myosin IgG antibodies in myocarditis/DCM and their cross-reactivity with the beta-adrenergic receptor.6-9 The purpose of this study was therefore to identify new biomarkers in early stages of HF in individuals who did not recover ejection fraction over a year and who were candidates for current immunotherapies.
METHODS
Patients with myocarditis and HF (N=41) within 6 months of onset were enrolled and monitored for 12 months. Serum autoantibodies and cytokines were detected by ELISA, and fluorescence-activated cell sorting analysis was performed on peripheral blood mononuclear cells. The Mann-Whitney U test was used for statistical analysis. For gene expression studies, blood from 10 DCM patients and 19 healthy controls were collected for RNA sequencing and transcriptomic analysis by Ingenuity Pathway Analysis (IPA®) and Reactome.
RESULTS
The results of the study correlate anti-human cardiac myosin autoantibodies with poor outcomes and demonstratedthat they functionally act on cardiomyocytes to activate protein kinase A. Concomitantly, a Th17 immunophenotype was significantly elevated in the blood as well as in cardiac biopsies.6 CD4+IL17+ T cells (p=0.0008), TGF-β (a Th17-promoting cytokine (p<0.0001), IL-6 (p<0.0001), IL-23 (p=0.0001), granulocyte-macrophage colony-stimulating factor (GMCSF) (p=0.0336), and GMCSF-secreting CD4+ T cells (p=0.0006) were significantly elevated in the blood.6 A Th17 immunophenotype was significantly associated with HF in males (p=0.029).6 Persistent HF (New York Heart Association [NYHA] functional classification III and IV) and non-recovery of left ventricular function were associated with significantly higher percentages of IL-17A-producing T cells at baseline, 6 and 12 months after onset, and IL-17A (p=0.019) and Th17-promoting cytokines IL-6 (p=0.0001) and TGF-β (p=0.0076).6 Regulatory T cells were significantly decreased (p=0.0006) and correlated with elevated Th17 cytokines in HF.⁶ Overrepresentation analysis of differentially expressed genes (adjusted p<0.05) in blood of DCM (>1 year) patients was carried out and revealed significant (false discovery rate=1.52E-13) enrichment of neutrophil degranulation (48 genes) in IL-8, GMCSF, IL-17A, and other pathways. Neutrophil involvement is a feature of the IL-17A pathways which promote fibrosis.
CONCLUSION
In conclusion, the study illustrated a strong Th17 signature (Figure 1) in more severe HF early in the disease with anticardiac myosin autoantibodies highly elevated in non-recovery of left ventricular function. Additionally, a strong correlation with neutrophil degranulation pathways in the later disease was observed, which could become biomarkers of fibrosis progression and disease severity in patients with HF. HF identified with a Th17 signature might be treated with immunomodulatory therapies such as anti-IL-17A.
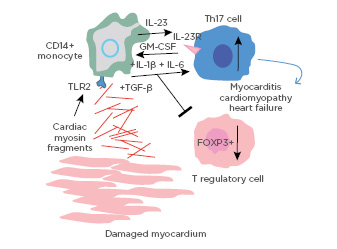
Figure 1: Diagram of the pathogenic mechanisms in the Th17 signature of myocarditis and dilated cardiomyopathy: an immunophenotype in myocarditis, dilated cardiomyopathy, and heart failure.FOXP3+: Forkhead box P3+; GM-CSF: granulocyte-macrophage colony-stimulating factor; TGF-β: transforming growth factor beta; TLR2: toll-like receptor 2.
Adapted from Myers JM et al.6








