Abstract
Little is known about the association between obstructive sleep apnoea (OSA) and vasospastic angina. The authors report the case of a 47-year-old female with vasospastic angina who had a high symptom burden despite nitroglycerine and a calcium channel blocker. After a sleep study revealed severe OSA, the patient was started on continuous positive airway pressure. Following 8 weeks of continuous positive airway pressure, the patient reported remarkable improvement in their symptoms. This case suggests an under-recognised link between OSA and vasospastic angina. While further clinical research is required, screening and treatment of OSA may be beneficial for patients with treatment refractory vasospastic angina.
Key Points
1. Vasospastic angina is an under-recognised cause of chest pain that affects females more commonly than males. Obstructive sleep apnoea (OSA) has been associated with nocturnal angina. However, the possible association between OSA and vasospastic angina is not well understood.
2. The authors report the case of a 47-year-old female with vasospastic angina who experienced debilitating symptoms despite nitroglycerine and a calcium channel blocker. A sleep study revealed severe OSA. They experienced remarkable improvement in their symptoms following the initiation of continuous positive airway pressure therapy.
3. OSA can worsen vasospastic angina via numerous mechanisms, including increased sympathetic tone and endothelial dysfunction. While further research is required, screening for OSA may be beneficial for patients with treatment refractory vasospastic angina.
PRESENTATION
A 47-year-old perimenopausal female had four presentations to the emergency department with acute chest pain syndrome. The chest pain occurred immediately after light physical activity, such as housework or walking two blocks. The pain radiated into their jaw, and was provoked by stress, fatigue, and cold weather. The patient’s medical history included hypertension, dyslipidaemia, a mildly elevated BMI of 27 kg/m2, migraines, and depression. From a reproductive perspective, menarche occurred at age 12 years and they were approaching menopause with increasing cycle irregularity. The patient had two previous pregnancies and was not on oral contraceptives. There was no family history of coronary artery disease. They were a lifelong non-smoker and consumed one alcoholic drink per week. Medications included lansoprazole and duloxetine. They were not on any sedatives or narcotics. The patient responded to nitroglycerine spray in the emergency department, but serial troponins and ECGs were negative. Their symptoms were frequently attributed to anxiety, and they were discharged home.
ASSESSMENT
The patient continued to experience chest pain on a weekly basis, including waking up with angina at night. In fact, the patient reduced their work hours due to the burden of their symptoms. The patient re-presented to the emergency department, where initial vitals included an elevated blood pressure of 158/96 mmHg, heart rate of 83 beats per minute, and O2 saturation of 99% on room air. Serial troponins were negative and ECG changes were limited to transient T wave inversions in lead V2. Chest X-ray, D-dimer, liver enzymes, lipase, and bilirubin were all within normal limits. The patient was discharged home with an antihypertensive and Cardiology follow-up.
The patient underwent further outpatient investigations as the cause of their chest pain remained unclear. An echocardiogram demonstrated preserved left ventricular function with an ejection fraction of 72% by the Simpson method, no regional wall motion abnormalities, normal right ventricular function, right ventricular systolic pressure of 25 mmHg, no significant valvular disease, and no pericardial effusion. A Holter monitor did not identify any significant abnormalities. Oesophagogastroduodenoscopy was negative and pulmonary function tests were normal.
DIAGNOSIS
The differential was narrowed to coronary artery disease versus uncontrolled hypertension. The patient underwent a coronary angiogram, which only demonstrated a 30–40% left anterior descending coronary artery stenosis (Figure 1). Their antihypertensives were titrated up to achieve a systolic blood pressure of 120–130 mmHg. However, they continued to have chest pain, prompting consideration of atypical causes of chest pain. Ultimately, the clinical presentation, including association of the chest pain with exposure to cold weather and often immediately after exercise, as well as a positive response to nitroglycerine, led to the presumed diagnosis of vasospastic angina.
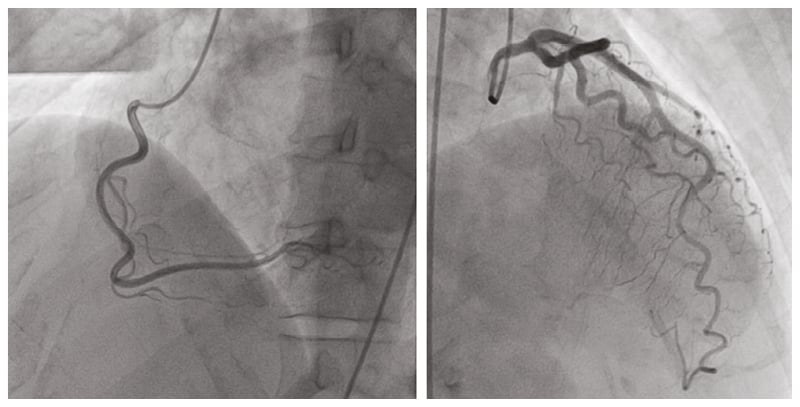
Figure 1: Coronary angiogram revealed subclinical coronary artery disease with a 30–40% left anterior descending coronary artery stenosis.
Standard treatment for vasospastic angina was initiated. The patient responded well to nitroglycerine patches, but use was limited by severe headaches. Therefore, diltiazem was titrated up to 360 mg daily and a nitroglycerine spray was prescribed for use on an as needed basis. While the patient experienced significant improvement, they continued to have symptoms, including nocturnal episodes, and were requiring nitroglycerine spray on a weekly basis.
After endorsing a history of fatigue, frequent headaches, and gasping for air at night, the patient was sent for a sleep study. They completed the Epworth Sleepiness Scale (ESS), which is a self-administered questionnaire to assess daytime sleepiness on a scale of zero to 24, and were found to have an elevated score of 11, consistent with excessive sleepiness.1 Based on the sleep study, the apnoea-hypopnoea index (AHI) was severely elevated at 43.5 events per hour, and the O2 desaturation index was severely elevated at 60.3 events per hour. The patient was diagnosed with obstructive sleep apnoea (OSA) and started on continuous positive airway pressure (CPAP).
MANAGEMENT
They were reassessed after 8 weeks of CPAP. The AHI was reduced to 3.9 events per hour with CPAP, and they now scored zero on the ESS. The patient endorsed remarkable improvement in their chest pain. They no longer experienced nocturnal angina, which previously occurred at least once per week. The patient also reported less daytime angina, only requiring nitroglycerine spray twice in the previous 8 weeks. Furthermore, the patient endorsed increased exercise capacity, and was able to walk up to 10 km per day without any post-exercise chest pain since starting CPAP therapy.
The authors hypothesise that the patient’s vasospastic angina was exacerbated by underlying OSA. Invasive testing for coronary vasospasm was considered but not performed. The authors’ centre, like many others, does not perform provocative testing due to the high risk of myocardial ischaemia and arrhythmias from uncontrolled, pharmacologically-provoked vasospasm. Unexpectedly, CPAP therapy had a remarkable effect on the patient’s angina burden.
Cardiovascular disease among females is inadequately researched, diagnosed, and treated.2 Vasospastic angina is likely an under-recognised cause of chest pain that affects females more frequently than males.3 OSA has been associated with nocturnal angina.4,5 However, the possible link between OSA and vasospastic angina is not well investigated. Some reports suggest that OSA is more common in those with vasospastic angina.6,7 In fact, Tamura et al.6 found that moderate-to-severe OSA (defined as AHI ≥15 events per hour) was independently associated with vasospastic angina (odds ratio: 9.61, 95% confidence interval: 2.11–43.78; p=0.003). Furthermore, within the context of limited literature, it appears that OSA exacerbates vasospastic angina. Teragawa et al.7 identified a trend towards more anginal attacks in the OSA group (2.9±0.6 per month) compared to the non-OSA group (1.3±0.8 per month; p=0.09). There was also a statistically significant difference in the frequency of intractable vasospastic angina among the OSA group (47%) relative to the non-OSA group (19%; p<0.05).7
There are a number of theories regarding the relationship between OSA and vasospastic angina.
Vasospastic angina involves transient coronary vasoconstriction that can lead to myocardial ischaemia.7 On the other hand, OSA is characterised by recurrent airway obstruction that leads to hypoxia, hypercapnia, and sleep fragmentation.8
OSA has been correlated to higher levels of pro-inflammatory cytokines that augment calcium sensitisation of vascular smooth muscle, predisposing to contraction.6 Additionally, increased sympathetic nervous system activity with arousal from sleep increases the tone of coronary arteries.8,9 Repetitive hypoxaemia, such as in the setting of recurrent airway obstruction, may also lead to the production of reactive O2 species and contribute to endothelial dysfunction.6,9 Finally, in animal models, the process of hypoxia and reoxygenation was associated with higher levels of the vasoconstrictor prostaglandin H2, and rendered bovine coronary arteries less responsive to the vasodilatory effects of nitric oxide.10
There may also be sex-specific mechanisms at play. The prevalence of vasospastic angina has been reported to be higher in females, especially with increasing age.3,11 A lower level of oestrogen in the post-menopausal state has been associated with increased inflammation and sympathetic nervous system activation, both of which would contribute to a favourable environment for vasospasm.12
This case highlights the association between OSA and vasospastic angina. While the authors’ patient experienced some improvement with antianginal therapy, their symptoms still had a significant impact on their quality of life. Unexpectedly, CPAP therapy provided the patient with substantial relief. Vasospastic angina is likely an under-diagnosed cause of chest pain among females. The combination of increased sympathetic tone, reactive O2 species, pro-inflammatory cytokines, and endothelial dysfunction may explain how OSA can worsen vasospastic angina. Providers should consider screening patients with vasospastic angina for symptoms of OSA, as CPAP therapy may improve symptom burden.







