Abstract
The original Fontan procedure was first introduced in the 1970s. The concept behind this surgical technique was revolutionary. It has subsequently transformed the lives of children born with complex congenital heart disease which was once thought to be inoperable and resulted in early death. The procedure itself has had several modifications over the decades, with subsequent improvements in long-term outcomes for these patients. Fontan patients are now surviving well into adulthood and the majority are able to live wholesome fulfilling lives. There are, however, a small proportion who are faced with the negative long-term physiological effects of this unconventional circulation. Early detection and management of these patients is the key to their long-term survival.
INTRODUCTION
As recently as the 1970s, congenital heart disease was predominantly a disease of childhood. With advances in surgical techniques, cardiopulmonary bypass, anaesthetics, and critical care medicine, nearly 85% of children with cardiovascular anomalies are expected to reach adulthood.1 By the year 2000, there were approximately equal numbers of adults and children with severe congenital heart disease.2,3 As these individuals with complex congenital heart disease reach adulthood, we are developing an increased understanding of the long-term sequelae of these early repairs.
THE FONTAN OPERATION
The Fontan operation is the definitive surgical palliation for patients with congenital heart disease in which septation into a biventricular system is not possible. This includes tricuspid atresia, double inlet left ventricle, hypoplastic left heart syndrome, and pulmonary atresia with intact septum. These diagnoses represent the most severe end of the spectrum of congenital heart disease and until the 1970s these patients usually died at a young age. The Fontan operation was first undertaken in 1968 in a patient with tricuspid atresia and was later described by Fontan and Baudet in 1971.4 It has transformed the outlook for patients with previously inoperable congenital heart disease.
The Fontan circulation allows for systemic venous blood to pass directly into the pulmonary circulation without the need for a subpulmonary ventricular pump (Figure 1). Flow through the lungs is therefore passive and is dependent on increased systemic venous pressure, active diastolic ventricular filling, and the effect of respiration to help move blood into and out of the pulmonary vasculature.
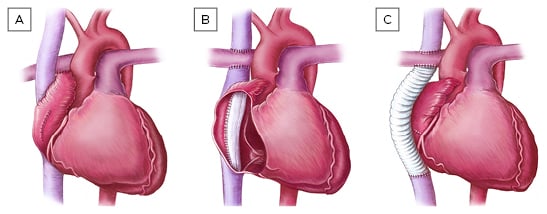
Figure 1: Different types of Fontan procedure.
A) atriopulmonary Fontan; B) lateral tunnel Fontan; C) extracardiac Fontan.
Not all patients, however, are suitable to undergo Fontan surgery and choosing the appropriate patient is a highly selective process. The recommendations for successful Fontan surgery have been refined since the procedure was first introduced but the general principles remain the same. An unobstructed connection between the systemic veins and the pulmonary artery, and patent pulmonary veins is a prerequisite. The ventricle itself should have a competent atrioventricular valve, outflow valve, and good systolic function, and the pulmonary arteries should be of good size with normal pulmonary vascular resistance.5
EVOLUTION OF THE FONTAN PROCEDURE
Over the last four decades, there have been modifications to the Fontan procedure in an attempt to further improve survival and minimise long-term complications. The original Fontan surgery was the atriopulmonary connection in which the right atrial appendage was anastomosed to the main pulmonary artery (Figure 1A). This technique was complicated by progressive right atrial dilatation, frequent atrial arrhythmias, and serious thromboembolic complications. The atriopulmonary Fontan has subsequently been superseded by the lateral tunnel Fontan (Figure 1B) and the extracardiac Fontan (Figure 1C). Both of these techniques achieve a total cavopulmonary anastomosis.6 The Fontan procedure is usually performed as a staged repair. The first stage is known as the bidirectional Glenn procedure or a hemi-Fontan. This involves redirecting superior vena caval flow directly to the pulmonary circulation whilst the inferior vena cava remains connected to the heart. The use of this staged technique avoids rapid volume loading on the pulmonary circulation.7
The lateral tunnel Fontan uses a baffle within the right atrium to directly channel the inferior vena cava to the right pulmonary artery and was first introduced in 1988.6 Like the atriopulmonary Fontan, this option also uses a right atriotomy approach and therefore can be subject to atrial arrhythmias. The extracardiac conduit on the other hand uses synthetic, usually Gore-Tex® tubing to connect the disconnected inferior vena cava to the right pulmonary artery. This technique was first introduced in 1990. The theoretical advantage of this technique is the avoidance of right atrial incisions and suture lines. It also avoids significant pressure loading and therefore dilation of the right atrium. The removal of these arrhythmic substrates is expected to reduce the propensity for intra-atrial re-entry tachycardia (IART).8 The extracardiac Fontan has subsequently become the technique of choice at many centres and has been the sole method of Fontan completion performed in Australasia since 2007.9
SURVIVAL
The perioperative mortality rate for initial Fontan surgery has decreased steadily over time. A large single-centre study from Boston, Massachusetts, USA, described a perioperative mortality rate of 36.7% for a first Fontan surgery performed before 1982 compared with 1.9% if performed in 1990 or later.10 These figures are partly a reflection of the type of Fontan procedure used in that era, partly increased surgical and anaesthetic expertise, and partly dueto an improvement in the understanding of optimal early postoperative care following this procedure. The recently published Australasian multicentre results show a perioperative mortality rate of 8% for initial Fontan surgery carried out before 1990 compared with 1% for those operated on after 2001. This comparatively low perioperative mortality rate in the earlier era may be partially explained by the somewhat more recent cohort and also by the more conservative patient selection in Australasia at the time.9
The Mayo Clinic, Rochester, Minnesota, USA, recently reported their overall 10, 20, and 30-year survival after the Fontan operation as 74%, 61%, and 43%, respectively. The centre found that operations carried out prior to 1991, use of preoperative diuretics, asplenia, and lack of preoperative sinus rhythm were all factors associated with decreased survival. Individuals with an extracardiac Fontan had a significantly better 30-year survival of 62% compared with the 46% and 39% for those with atriopulmonary and lateral tunnel Fontans, respectively.10
The Australasian figures showed an overall survival estimate at 15, 20, and 25 years as 93%, 90%, and 83%, respectively. The 25-year survival for the atriopulmonary Fontan was 76% compared with a 90% survival for the lateral tunnel at 20 years and a 97% survival for the extracardiac Fontan at 13 years.11 Although the survival estimates appear better when compared with cohorts elsewhere, this was a somewhat later cohort and follow-up times were shorter for the lateral tunnel and extracardiac Fontan.
Death in the Boston group’s Fontan population was found to predominantly occur perioperatively (68.4%) followed by sudden death (9.2%), thromboembolism (7.9%), heart failure (6.7%), sepsis (2.6%), and other (5.2%). Sudden death was presumed to be largely arrhythmic in origin.12 The Mayo Clinic reported similar aetiologies for late death.13 Independent risk factors for heart failure death were protein losing eneteropathy, single morphological right ventricle, and higher right atrial pressure.12
QUALITY OF LIFE
Fontan patients have a significantly reduced exercise capacity compared with healthy subjects.14-16 Patients with total cavopulmonary circulation had an exercise capacity reaching only about 60% of their reference values and only one-third of Fontan patients had a peak VO2 within the normal range.17,18
Fontan patients, however, generally have a good perception of their own health status.18,19 Eighty percent of adult Fontan patients rated their own health as excellent and a similar proportion thought their physical status was improved post-Fontan surgery.11 In a Danish case control study, more than half of the Fontan patients were leading a normal life and were able to work or study full-time. A quarter of the patients had mild symptoms and were still able to work part-time.20 Australian data on Fontan patients who were arrhythmia free reported that despite having restricted exercise capacity, patients had a normal quality of life in their reports of psychiatric symptoms and personal relationships.21 The majority of Fontan patients have an overall good quality of life and are largely unaffected by their cardiac diagnosis.
PREGNANCY
The physiological changes of pregnancy, namely increased blood volume, chronotropic incompetence, and compression of the inferior vena cava in the gravid state can all compromise the finely balanced Fontan circulation. There is a tendency for arrhythmias and thrombosis in Fontan patients and the likelihood of this is further increased during pregnancy.22
Pregnancy in the Fontan population has previously been strongly discouraged but this opinion has gradually changed over time. The number of Fontan patients at any one centre who have undergone a pregnancy remains limited and therefore so too does the literature. In 2006, Drenthen et al.23 reported that Fontan patients were able to successfully complete a pregnancy without any long-term sequelae. This was however at the expense of other complications, namely deterioration in functional status, atrial fibrillation, gestational hypertension, premature rupture of membranes, premature delivery, fetal growth retardation, and neonatal death. Fifty percent of patients in this small cohort had miscarriages. This is supported by more recent data reporting that pregnancy in Fontan patients is associated with a high rate of miscarriage, preterm delivery, and low birth weight.24 Another recent study of 59 pregnancies in 37 Fontan patients, one of the largest to date, found there was a cardiac complication rate of 10%, with atrial arrhythmias being the most common followed by thromboembolism and heart failure. There were no maternal deaths. Twenty-seven percent of these pregnancies however ended in miscarriage, predominantly occurring in the first trimester.25
Pregnancy outcomes in Fontan patients can potentially be favourable, however this may be compromised by the presence of cardiac and extracardiac complications of the Fontan circulation. Fontan patients therefore need individualised pre-pregnancy counselling and advice on both maternal and fetal outcomes. This should take into account their functional status, ventricular function, arrhythmia burden, comorbidities, and objective exercise data wherever possible.26 These patients will need close monitoring throughout the pregnancy and ideally at a specialised tertiary level hospital.
LONG-TERM COMPLICATIONS
Arrhythmias
Sinus node dysfunction has been reported in up to 40% of patients with atriopulmonary connections and in 25% of those with lateral tunnel or extracardiac connections.27,28 The haemodynamic consequence of non-sinus rhythm in the Fontan population can be marked, as the contribution of atrial systole to ventricular filling contributes significantly to optimising forward flow through the pulmonary vasculature. Loss of sinus rhythm may therefore increase pressure within the Fontan circuit and central veins thereby also reducing cardiac output.
Atrial arrhythmias are not infrequent in the Fontan population and are driven by elevated right atrial pressures and the effects of direct surgical intervention on the right atrium. The more recent modifications to the Fontan procedure have however resulted in a lower incidence of atrial arrhythmias. In the Australia and New Zealand Fontan Registry, predictors for supraventricular tachycardia were found to include atriopulmonary and lateral tunnel Fontan, compared with the extracardiac Fontan.11 A study from the Royal Brompton Hospital showed that right atrial size was the strongest predictor of IART in those with the atriopulmonary Fontan.29 The extracardiac Fontan meanwhile maintains low pressure in the right atrium thus avoiding atrial dilation. This is likely to explain the lower incidence of atrial arrhythmias in this group.8
Episodes of IART may lead to rapid haemodynamic decompensation in the setting of a Fontan circulation and may also represent a high risk of thrombus formation within the dilated right atrium. The termination of an acute episode of atrial tachycardia within 24 hours from onset is therefore recommended. Ventricular arrhythmias are rare in Fontan patients and if seen, are in the context of severe ventricular dysfunction.
Thromboembolism
Fontan patients are at an increased risk of thromboembolism compared with the general population due to a combination of factors including: diminished cardiac output, absence of pulsatile flow in the pulmonary arteries, abnormal patterns of venous flow, and the presence of prosthetic material. There have also been several reports of clotting factor abnormalities and increased platelet reactivity in Fontan patients.30,31 Despite this, the Australia and New Zealand Registry found that 82% of Fontan patients were free from overt thromboembolic events at 25 years.10 Currently, treatment strategies to prevent thromboembolism vary between institutions.
Khairy et al.12 demonstrated thromboembolism as a cause of late death in Fontan patients in the absence of antiplatelet agents or anticoagulation. Meanwhile, Robbers-Visser et al.32 found no difference in thromboembolism between those with a lateral tunnel Fontan circulation and those with an extracardiac conduit. This was also irrespective of the postoperative use of antiplatelet agents or anticoagulants.
A recent meta-analysis of 10 studies however found that there was a significantly lower incidence of thromboembolism in Fontan patients if either aspirin or warfarin was used. There was also no significant difference in thromboembolic events in patients receiving aspirin compared with warfarin. The results were similar when the seven studies using the newer-generation Fontan patients were looked at exclusively.33 Although this data suggests that there is no clear benefit of warfarin over aspirin, the event rate is likely to be low and occur over a long period of follow-up, making it difficult to draw definite conclusions.
The use of warfarin in clinical practice however is highly variable and hard to accurately represent in studies. Additionally, one study found aspirin resistance in up to 52% of adult Fontan patients.34 Therefore although aspirin does appear to be a reasonable alternative to warfarin, the results of this meta-analysis may not necessarily change the way of practice in many centres just yet. The use of novel anticoagulants in the Fontan population is yet to be studied but is undoubtedly an area of great promise for this relatively young patient population, who may find compliance with international normalised ratio testing difficult.
Ventricular Dysfunction
Progressive ventricular systolic dysfunction and atrioventricular valve sufficiency are late complications in Fontan patients. Eicken et al.35 used cardiac magnetic resonance imaging to show a progressive decline in ventricular systolic function at 10-year follow-up, with a median ejection fraction of 49.3% (range 20–63%). The Boston group found that the presence of a pacemaker before Fontan operation was associated with an increased probability of late failure whilst the morphology of the ventricle itself was not a predictor.36
Diastolic dysfunction was assessed in a small cohort of Fontan patients: 57% were noted to have diastolic dysfunction, 7% had impaired relaxation, 29% had pseudonormalisation, and 21% had restrictive physiology. Those with diastolic dysfunction were noted to have a lower peak VO2 on exercise testing.37 Diastolic dysfunction and reduced ventricular compliance, if present, was seen to persist even late after Fontan surgery.38
Complications Related to High Venous Pressure
The Fontan circuit allows for passive venous flow into the lungs via the vena cava without pulsatile ventricular contraction. As a result, the systemic venous pressure in a Fontan circulation remains obligatorily elevated. Venous congestion and decreased perfusion are the hallmarks of this circulation. The resultant long-term complications from such a circulation include protein-losing enteropathy and plastic bronchitis. Both of these complications have been regarded as representing a ‘failing’ Fontan circulation and are associated with a high risk of adverse outcomes, including death in the short-to-medium term.
Liver Disease
Liver disease is being reported with increasing frequency in adult Fontan patients and was previously only reported in small cohorts.39-41 Fontan-associated liver disease is thought to be multifactorial and begins with the onset of multiple liver insults during childhood. This includes episodes of hypoxaemia and numerous perioperative episodes of liver injury. In adulthood, chronic venous congestion and diminished cardiac output lead to reduced portal flow and portal vein saturation. This impairs the liver’s ability to autoregulate its own blood flow and liver disease ensues.42
The pattern of liver injury in adult Fontan patients is similar to other forms of cardiac cirrhosis in both its gross and histological features.42,43 The severity of Fontan-associated liver disease has been found to correlate best with the duration of the Fontan circulation and hepatic venous pressure.39 The routine screening of the liver in Fontan patients is now recommended, although the optimal imaging modality in this subset of patients is yet to be determined.
MANAGEMENT OF THE ‘FAILING’ FONTAN
The ‘failing’ Fontan refers to the physiological deterioration of the Fontan circulation and the consequential extracardiac manifestations that follow. Therapeutic options for the management of the failing Fontan are in general limited to Fontan conversion surgery or heart transplantation.
Fontan Conversion
As previously discussed, the outcomes following the lateral tunnel and extracardiac Fontan appear to be superior to those following the atriopulmonary Fontan. This has led various groups to perform surgery to convert patients from the atriopulmonary Fontan circulation to a total cavopulmonary connection which today would be the extracardiac Fontan. Fontan conversion surgery was first championed by Mavroudis et al.44 with good results. A transatlantic multicentre study later published by Marcelletti et al.45 found that Fontan conversion surgery could be performed with relatively low morbidity and mortality, whilst improving functional class.
In most centres the predominant indication for Fontan conversion surgery has been recurrent atrial arrhythmias. Radiofrequency catheter ablation of IART in the adult Fontan population has been found to have a higher incidence of recurrence compared with other forms of congenital heart disease.46 Therefore arrhythmia surgery in the form of a right atrial +/- left atrial Maze procedure is often combined with Fontan conversion surgery. Many groups also incorporate pacemaker implantation. The Chicago group found promising results with this approach. As well as an improvement in both New York Heart Association (NYHA) class and exercise tolerance, follow-up at 15 years showed 80% freedom from death or transplantation and 85% freedom from recurrent tachycardia.47-49
Cardiac Transplantation
The appropriate time to list adult Fontan patients for cardiac transplantation is highly controversial. Survival following transplantation after a previous Glenn or Fontan procedure in adults was reported as 71.5% at 1 year and 67.5% at 5 years. The early perioperative mortality rate in the Fontan patients was however 37.5% and was predominantly due to haemorrhage and infection. Of those with pre-existing protein-losing enteropathy, 40% died within 6 weeks post-transplant. In those who survived the first year however, the late mortality rates were not significantly different when compared with patients transplanted for other aetiologies.50,51
In adult Fontan patients, the development of haemodynamic abnormalities and extra-cardiac complications are often gradual and insidious. The detection of a failing Fontan is complicated by chronic functional limitation and the lack of new symptoms in many patients. Identifying at-risk Fontan patients before they develop irreversible multi-organ dysfunction is the key to improving long-term outcomes in these patients and early listing should be considered for patients with evidence of hepatic dysfunction. The 2008 Adult Congenital Heart Disease guidelines give a Class IIb indication for heart transplantation for severe ventricular dysfunction or protein-losing enteropathy in Fontan patients.52 There are at present no well-established criteria to assist with the timing of transplant listing for adult patients with Fontan failure and the development of such guidelines will be the key to the successful ongoing management of these patients.53-57
CONCLUSION
The Fontan operation is one of the most innovative techniques in the congenital cardiac surgical repertoire and has transformed the outlook for children born with complex congenital heart disease. A large proportion of adult Fontan patients are able to enjoy a good quality of life and are largely unaffected by their cardiac diagnosis. There is however a small minority who are faced with the long-term negative physiological consequences of this circulation, manifesting as both cardiac and extra-cardiac sequelae. Meticulous monitoring of these patients will allow for optimisation of their cardiac status whenever possible as well as early referral for either Fontan conversion surgery or cardiac transplantation when indicated.








