Abstract
It has long been established that elevated plasma concentrations of low-density lipoprotein cholesterol (LDL-C) are among the prominent contributors leading to the development of atherosclerotic plaques and, ultimately, cardiovascular disease. In the current era of optimal risk factor modification, proprotein convertase subtilisin/kexin type 9 (PCSK9) targeting has emerged as a potent therapeutic approach in the management of hypercholesterolaemia, addressing several substantial, unmet clinical needs. PCSK9 monoclonal antibodies, evolocumab and alirocumab, as well as inclisiran, which is a small interfering RNA that halts the transcription of PCSK9 mRNA, are being increasingly used in current clinical practice, as they induce intensive LDL-C reductions without any significant safety and tolerability concerns. Based on the success of these agents, the concept of PCSK9 targeting with novel agents with enhanced biological properties, or via different administration routes, has received considerable attention. In this regard, numerous antisense oligonucleotides, peptides, and proteins are currently under evaluation in randomised controlled trials, yielding propitious results up to date; they may enter clinical use in the coming years. Meanwhile, a PCSK9 vaccine, as well as genome editing via clustered regularly interspaced palindromic repeats/Cas9, hold great promise to eradicate LDL-C altogether as a cardiovascular risk factor. This review aims to present and discuss the current clinical and scientific evidence pertaining to the field of medications that exert their biological effect by targeting PCSK9, which are either in use in clinical practice, or are currently being evaluated in pre-clinical or clinical studies, and may prove beneficial in the near future.
Key Points
1. Proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies and inclisiran have ushered in a new, exciting era of hypercholesterolaemia management, addressing several unmet needs.
2. Unprecedentedly low low-density lipoprotein cholesterol concentrations can be achieved with PCSK9 monoclonal antibodies and inclisiran, without any significant safety and tolerability concerns.
3. PCSK9 targeting with antisense oligonucleotides, as well as various peptides and proteins, are under evaluation in randomised clinical trials, having provided encouraging results.
INTRODUCTION
Cardiovascular disease (CVD) has consistently been the leading cause of disease burden in the world, and the reduction of CVD mortality consists a major public health challenge in most societies.1 Elevated low-density lipoprotein cholesterol (LDL-C) levels in the bloodstream are a well-established causative, modifiable risk factor for atherosclerotic plaque formation. It is estimated that 2.6 million deaths are attributed to raised cholesterol levels worldwide annually; hence, hypercholesterolaemia is rightly considered a silent epidemic by many researchers.2 In view of the above, lipid-modifying therapies are a fundamental pillar in current preventive cardiology practice.
Among the available pharmacologic agents, statins are an effective lipid-modifying therapy that has proved its clinical value over the last decades. Statins have become the mainstay of lipid-lowering therapy. Nevertheless, a significant percentage of patients (7–29%) experience statin-associated muscle symptoms, which, although mild in severity in most patients, are one of the principal reasons for statin non-adherence and/or discontinuation, which inevitably contributes to adverse cardiovascular outcomes.3 Additionally, it has been demonstrated that statins exert a detrimental effect on glycaemic control, resulting in a significant increment of new-onset diabetes.4
The current era of preventive cardiology continues to emphasise the significance of LDL-C goal attainment of each patient, according to the individual risk stratification for CVD. However, cumulative evidence suggests that only a disappointing 20% of very high-risk patients achieve the recommended risk-defined LDL-C threshold.5 This phenomenon is also observed in patients with familial hypercholesterolaemia, further increasing their already increased CVD risk.6
Another intriguing phenomenon is that many patients remain at a significant residual CVD risk despite LDL-C goal attainment, reinforcing the need for the development of novel lipid-modifying agents.7 In this regard, the addition of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in the lipid-lowering armamentarium was monumental, irrevocably changing the scope of lipid-lowering treatment, and closing the gap in optimal LDL-C reduction. This is due to the fact that unprecedentedly low levels of LDL-C can be reached by PCSK9 inhibitors, which also possess a favourable safety and tolerability profile. Therefore, the concept of PCSK9 targeting via different mechanisms or administration routes has garnered considerable attention over the last decade. In this article, the authors will explore the field of medications that exert their biological effect by targeting PCSK9, which are either in use in clinical practice, or are currently being evaluated in pre-clinical or clinical studies.
WHY IS TARGETING THE PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9 PATHWAY SUCCESSFUL?
The impetus for the breakthrough discovery of the crucial role of PCSK9 as a pivotal regulator of LDL-C levels was initiated in 2003, when a novel gain-of-function missense mutation in PCSK9 was identified as a cause of familial hypercholesterolaemia and premature CVD.8 Conversely, loss-of-function mutations in PCSK9 were recognised to induce low LDL-C levels, providing substantial protection against CVD to their carriers.9
The mechanism through which PCSK9 exerts its biological effect on LDL-C homeostasis has now been fully elucidated. PCSK9 is a serine protease predominantly derived from hepatocytes and, to a lesser extent, from the small intestine, kidney, pancreas, and immune system. PCSK9 possesses the ability to bind to the low-density lipoprotein receptor (LDL-R), leading to its lysosomal degradation in a non-enzymatic fashion, thus decreasing the availability of LDL-R on the surface of the hepatic cell membrane. As a result, the removal rate of LDL-C from the bloodstream is decreased.10 Therefore, the pharmacological inhibition of PCSK9 function became an attractive treatment strategy to lower circulating LDL-C levels substantially.
Recent evidence suggests that the function of PCSK9 may not be limited to LDL-C metabolism. On the contrary, there are indications that the inflammatory response, platelet aggregation, and thrombosis, as well as neuronal apoptosis, may be mediated to some extent by PCSK9.11 Additionally, there is an indication of a linear association between high circulating PCSK9 levels and adverse cardiovascular outcomes.12
PROPROTEIN CONVERTASE SUBTILISIN/KEXIN TYPE 9 MONOCLONAL ANTIBODIES
The approval of the first PCSK9 inhibitor from the U.S. Food and Drug Administration (FDA) in 2015 came to address several unmet needs in the management of hypercholesterolaemia and, ultimately, CVD. Today, alirocumab and evolocumab have come to represent valuable additions to the lipid-lowering armamentarium used in current clinical practice.
The beneficial properties of PCSK9 inhibitors have been substantiated by numerous studies to date. The most notable studies, which evaluated the impact of PCSK9 inhibitors on cardiovascular outcomes, were the ODYSSEY LONG TERM13 and FOURIER14 trials.
In ODYSSEY LONG TERM, which included 2,341 patients on background statin treatment at the maximum tolerated dose, treatment with alirocumab resulted in a decrement of LDL-C values by 52.4% after 78 weeks. Additionally, alirocumab led to a lower rate of major adverse cardiovascular events, thus providing evidence for the favourable cardiovascular outcomes deriving from PCSK9 inhibitors therapy.13 The above findings were confirmed by the FOURIER trial, which assessed the effect of evolocumab on 27,564 patients who were receiving statin treatment over a median follow-up period of 2.2 years. Treatment with evolocumab led to a significant reduction of the primary endpoint (cardiovascular death, myocardial infarction, stroke, hospitalisation for unstable angina, or coronary revascularisation) by 15%, as well as of the secondary endpoint (cardiovascular death, myocardial infarction, or stroke) by 20%. Additionally, the magnitude of the improved cardiovascular outcomes tended to increase over time.14
An open-label extension study of the FOURIER trial, FOURIER-OLE, which enrolled a total of 6,635 patients, indicated that LDL-C lowering with evolocumab was associated with persistently low rates of adverse events over a median follow-up period of 5 years, and maximum exposure time over 8 years.15 Meanwhile, mounting evidence suggests that PCSK9 inhibitors are a safe therapeutic intervention in patients with diabetes, which do not adversely affect glucose metabolism, as opposed to statins.16
Since the advent of PCSK9 inhibitors, a putative concern was that the very low achieved LDL-C levels may be associated with cognitive deficits or other significant side effects. However, current evidence suggests that attaining low LDL-C levels, even below 15 mg/dL, is safe and well-tolerated, and that cognitive function is not affected in patients receiving PCSK9 inhibitors after 96 weeks of treatment.17-19 Conversely, the robust decrease in circulating LDL-C values led to further improvement of cardiovascular outcomes, indicating that the concept of ‘the lower, the better’ regarding circulating LDL-C levels may be pursued in clinical practice.17 It has been demonstrated that for each 1 mmol/L (38.67 mg/dL) reduction in circulating LDL-C values induced by statin therapy, a 22% proportional reduction in the risk of major vascular events is achieved.20 However, relevant data regarding PCSK9 inhibitors therapy are lacking to date.
The approach of ‘the earlier, the better’ regarding the initiation of LDL-C lowering therapy has gained much attention over the last decade. Numerous studies have shown that the administration of PCSK9 inhibitors in patients with acute coronary syndrome as early as possible leads to improved cardiovascular prognosis; however, it warrants further evaluation by large-scale clinical studies, so that more reliable and concrete conclusions can be drawn.21
The realisation in numerous epidemiological and genetic studies that elevated levels of lipoprotein (a) [Lp(a)] are a causal and independent risk factor for CVD, has led to an intensive investigation into therapeutic strategies targeting Lp(a). Treatment with PCSK9 inhibitors induces reductions of approximately 25–30% in circulating Lp(a), a characteristic of paramount importance, taking into account the shortage of therapeutic interventions for hyperlipoproteinaemia (a) thus far.22 To date, lipoprotein apheresis remains the only therapy approved by the FDA for lowering Lp(a) levels in the USA. The exact mechanism of Lp(a) reduction by PCSK9 inhibitors, as well as their potential role in the management of hyperlipoproteinaemia (a) in clinical practice, remains to be elucidated.
A significant barrier limiting the widespread use of PCSK9 inhibitors that is yet to be overcome is their associated annual cost. The adverse cost-effectiveness of PCSK9 inhibitors, which in many countries is well beyond the highest acceptable willingness-to-pay threshold per quality-adjusted life years, renders them as not affordable for most healthcare systems or private insurance companies.23 Indeed, rates of prescription approval for PCSK9 inhibitor therapy by health insurance companies, even for patients who appeared to meet labelled indications, were extremely low.24 However, a more recent price reduction of 60% from their initial pricing,23 and the expected further reduction in price over time, will eventually allow more patients to receive PCSK9 inhibitors in the foreseeable future.
INCLISIRAN
One of the most exciting advances in biology has been the discovery of small interfering RNAs, mediating their action by selective post-transcriptional gene silencing. The advent of inclisiran, which is a long-acting, short-chain small interfering RNA, was a remarkable feat of drug development that ignited the dawn of a new era of lipid-lowering therapy. In contrast to the PCSK9 monoclonal antibodies, whose site of action lies on an extracellular level, inclisiran acts intracellularly by halting the hepatic synthesis of PCSK9 mRNA.25 Inclisiran is among the five approved small interfering RNA medications by the FDA; it is a drug discovery paradigm for the development of novel drugs.
In the ORION-1 Phase II trial, which was the benchmark for subsequent studies, a single dose of 500 mg inclisiran resulted in a reduction of circulating LDL-C levels by 41.9% at Day 180, whereas the two-dose regimen of 300 mg of inclisiran yielded a decrement of 52.6% in LDL-C levels in the same period. Moreover, inclisiran appeared to have an acceptable safety and tolerability profile, as the incidence of serious adverse events did not differ substantially between treatment and placebo groups.26
The above findings have been replicated in several more recent randomised, controlled trials. The most comprehensive evidence regarding the efficacy of inclisiran has been documented in two Phase III trials, ORION-10 and ORION-11.27 The studies comprised a total of 3,178 patients with elevated LDL-C levels, despite maximum-tolerated statin treatment. The follow-up period for both studies was 1.5 years. Administration of 284 mg of inclisiran subcutaneously every 6 months resulted in a 45.8–51.3% decrease in circulating LDL-C levels. Regarding the safety of inclisiran, mild injection site reactions were more common in the inclisiran group.27
Preliminary data on the long-term safety profile of inclisiran were provided by the ORION-3 study, a 4-year open-label extension study of ORION-1.28 The study indicated that the repeated exposure to inclisiran was not associated with the emergence of severe adverse events, and resulted in sustained reductions in LDL-C levels.28 Furthermore, it has been suggested that inclisiran is a safe therapeutic option in patients with mild or moderate hepatic impairment.29
Although evolocumab, alirocumab, and inclisiran exhibit similar potency in decreasing circulating LDL-C values, inclisiran offers the advantage of a considerably more convenient dosing regimen, as it is administered at 6-month intervals. This beneficial property of inclisiran has the potential to eradicate medication non-adherence and, consequently, to improve the rates of achievement of LDL-C goals in the foreseeable future.30
Another question to be answered is whether the beneficial characteristics of inclisiran are translated into improved CVD outcomes. Thus, the results of the ORION-4 study, which is the first trial investigating the effects of inclisiran on major adverse cardiovascular events, are eagerly awaited at the second half of 2026.31
A summary of the key clinical trials of PCSK9 monoclonal antibodies and inclisiran is shown in Table 1.
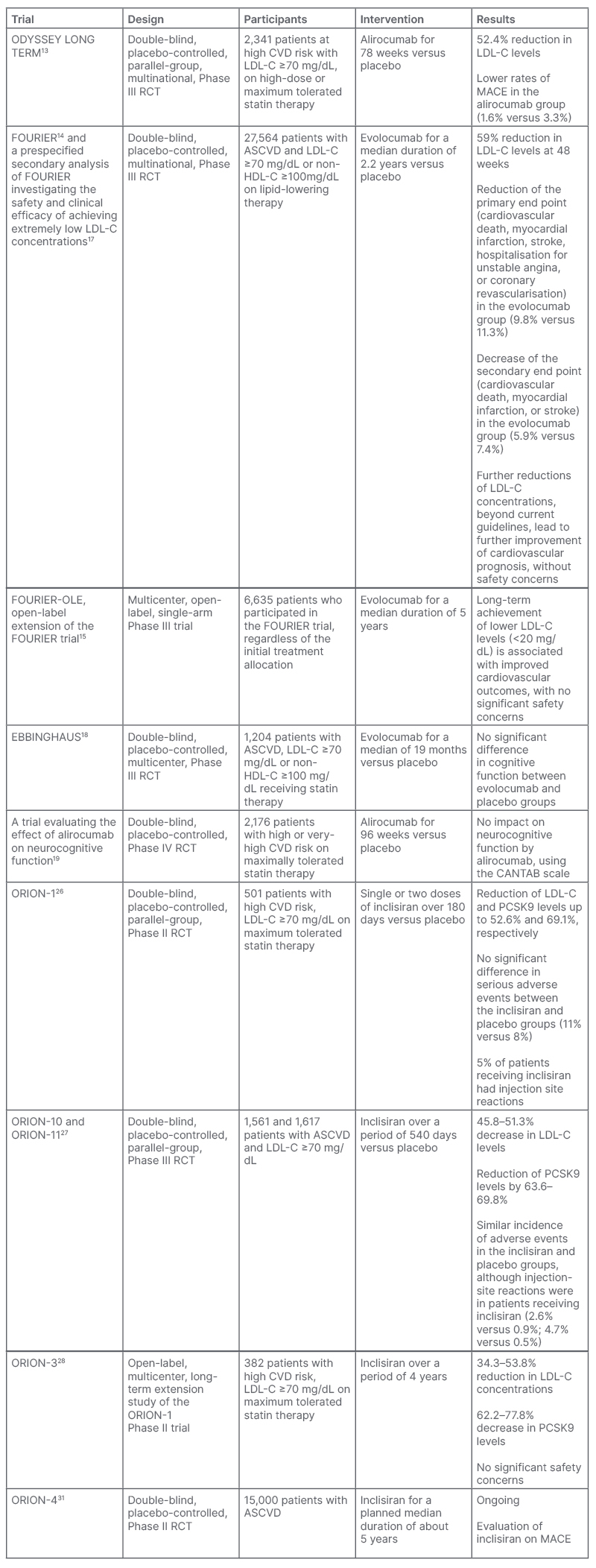
Table 1: Summary of key clinical trials of PCSK9 monoclonal antibodies and inclisiran.
ASCVD: atherosclerotic cardiovascular disease; CANTAB: Cambridge Neuropsychological Test Automated Battery; CVD: cardiovascular disease; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MACE: major adverse cardiovascular event; PCSK9: proprotein convertase subtilisin/kexin type 9; RCT: randomised controlled trial.
EMERGING THERAPIES: WHAT IS NEXT?
Although three PCSK9-targeting medications are currently approved, and are extremely valuable in the management of hypercholesterolaemia, the endeavour for the development of novel drugs that target PCSK9 has not yet ceased. On the contrary, several promising therapeutic interventions are under pre-clinical or clinical evaluation.
Antisense Oligonucleotides
Cepadacursen sodium (CiVi 007) and CiVi 008, which is the oral formulation of the subcutaneously administered CiVi 007, are long-acting, third-generation antisense oligonucleotides that inhibit the production of PCSK9 at the level of its synthesis by mRNA. CiVi 007 has the potential to combine robust LDL-C reduction with convenient dosing and an affordable price. It has completed a Phase I and Phase II trial, but the results have yet to become available.32 CiVi 008, the only oral antisense oligonucleotide in development targeting PCSK9, is now in the pre-clinical development stage, and the clinical trials are awaited with interest.
Although AZD8233 (ION449), an antisense oligonucleotide targeting PCSK9 gene expression, had provided promising results in a Phase II trial (73% reduction of LDL-C), it was announced that the manufacturing company would halt clinical studies due to lack of goal attainment.32
Peptides and Proteins
Lerodalcibep (LIB003) is a recombinant fusion protein of a PCSK9-binding domain, adnectin, and human serum albumin. Adnectin, the target-binding protein, attaches to PCSK9; therefore, the interaction with the LDL-R is impeded. In a Phase II clinical trial, the subcutaneous administration of LIB003 every month resulted in an approximately 60% reduction of circulating LDL-C levels over a 36-week dosing period, and had a benign side effect profile.33 The safety and efficacy of LIB003 are currently evaluated in Phase III clinical trials, and the results are awaited at the end of 2023.34
MK-0616, an oral macrocyclic peptide inhibitor of PCSK9, might be a revolutionary addition to lipid-lowering therapy. In a Phase IIb clinical trial comprising 381 patients, once-daily administration of MK-0616 resulted in clinically meaningful LDL-C reduction of up to 60.9%. Meanwhile, there was no association with the emergence of adverse events during the 8 weeks of treatment and additional 8 weeks of follow-up.35 Thus, MK-0616 holds the potential to become the first oral PCSK9 inhibitor, and to be a major player in hypercholesterolaemia management. A Phase III trial of MK-0616 is expected to be initiated in the second half of 2023.36
Numerous other drugs that exert their action by targeting PCSK9 are being evaluated in pre-clinical or early clinical studies. NNC0385-0434 is a small molecule peptide PCSK9 inhibitor administered orally, once-daily. It has a similar structure to LDL-R and inhibits PCSK9 binding to LDL-R. A Phase II clinical trial of NNC0385-0434 was completed in the first half of 2023, and the results are awaited.32 CVI-LM001 is a first-in-class oral small molecule PCSK9 modulator inhibiting PCSK9 transcription, and degradation of LDL-R mRNA. Preliminary clinical data show that CVI-LM001 reduces the expression level of the PCSK9 gene by 90%, and exhibits good pharmacokinetics.37
The addition of oral PCSK9 targeting agents to the lipid-lowering armamentarium may be particularly useful in patients who are not comfortable with injections, leading to improved treatment compliance, and eventually better cardiovascular prognosis.
Vaccines and Clustered Regularly Interspaced Palindromic Repeats/Cas9
Last but not least, a PCSK9 vaccine, namely AT04A, as well as genome editing via clustered regularly interspaced palindromic repeats/Cas9, hold great promise to transform the treatment landscape for patients with hypercholesterolaemia in the future.38-40
AT04A has completed a Phase I clinical trial providing encouraging results, while the approaches of PCSK9 gene editing are only at the in vivo stage of development.38
Future Hopes: Identification of New Molecular Targets
To date, there is intensive research for the identification of factors that may play a role in the regulation of LDL-C/PCSK9 homeostasis, which could lead to the recognition of novel mechanisms for LDL-C lowering. For instance, annexin A2 has been identified as a natural extrahepatic inhibitor of the PCSK9, while mice that are deficient in the protein denitrosylase S-nitroso-coenzyme A reductase 2 exhibit marked reductions in serum cholesterol, due to reduced secretion of PCSK9.41,42
Thus, alternative pathways might present exciting opportunities for the development of novel pharmacologic interventions.
An overview of the therapeutic interventions targeting PCSK9 that are currently in use, or are being evaluated in pre-clinical or clinical trials, is shown in Figure 1, and their mechanism of action is shown in Figure 2.
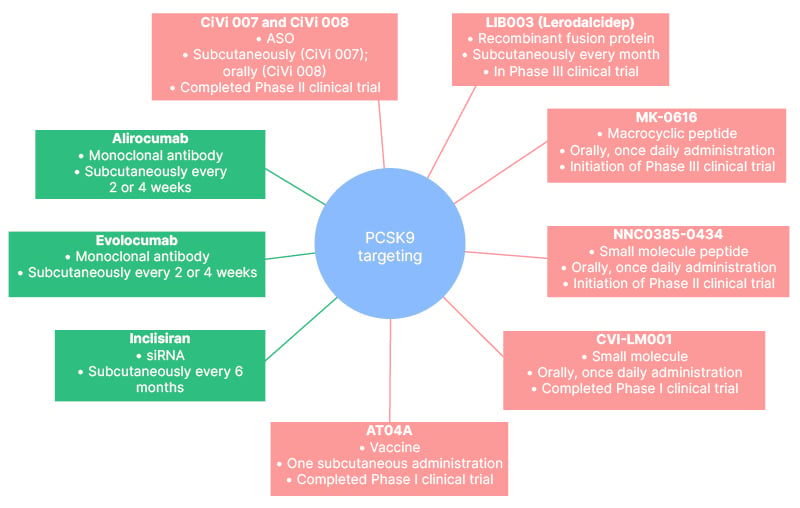
Figure 1: Therapeutic interventions targeting proprotein convertase subtilisin/kexin type 9.
ASO: antisense oligonucleotides; CiVi 007: cepadacursen sodium; CiVi 008: oral form of cepadacursen sodium; PCSK9: proprotein convertase subtilisin/kexin type 9; siRNA: small interfering RNA.
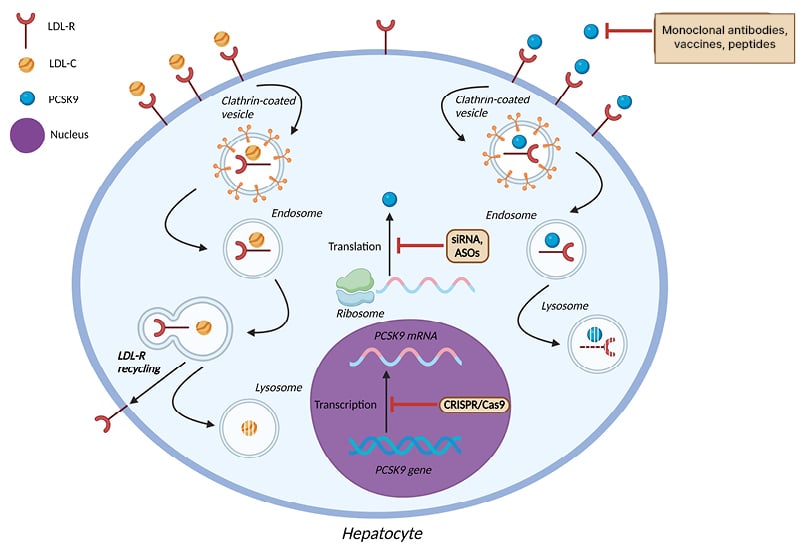
Figure 2: Mechanism of action of pharmacologic agents targeting proprotein convertase subtilisin/kexin type 9.
ASO: antisense oligonucleotides; LDL-C: low-density lipoprotein cholesterol; LDL-R: low-density lipoprotein receptor; PCSK9: proprotein convertase subtilisin/kexin type 9; siRNA: small interfering RNA.
CONCLUSION
Indisputably, the advent of medications that act via the targeting of PCSK9 has ushered in a new, stunning era of preventive cardiology and optimal risk factor modification. PCSK9 monoclonal antibodies and inclisiran, which are currently in use, have garnered an increasing reputation, capitalising on their potency and efficacy in decreasing both circulating LDL-C values and major adverse cardiovascular events, as well as on their favourable safety and tolerability profile. Novel pharmacological agents with enhanced pharmacokinetic and pharmacodynamic properties are being evaluated in clinical trials, having provided propitious results up to date. However, cardiovascular outcomes studies are eagerly awaited, so as to ascertain whether the marked reduction in LDL-C levels induced by these novel agents are also translated into improved cardiovascular prognosis. Additionally, large-scale trials evaluating the long-term safety and tolerability of these therapeutic interventions are awaited with interest. Another question that is yet to be answered is whether these agents would be tolerable in specific categories of patients, such as in those with renal or hepatic impairment, or in females who are pregnant with familial hypercholesterolaemia.
Considering all of the above, the future seems vivid for the medications that target PCSK9, with the optimal goal of eradicating hypercholesterolaemia as a cardiovascular risk factor via pharmacotherapy being closer than ever. Meanwhile, the success story of alirocumab, evolocumab, and, more recently, inclisiran, has paved the way for the development of novel therapies for a number of different diseases.