Abstract
An elderly hypertensive lady presented to us with acute coronary syndrome; an angiogram revealed total thrombotic occlusion of large left circumflex artery. After thrombosuction, there was proximal tight stenosis followed by an aneurysmal segment of the culprit vessel that was stented successfully. Subsequent post-dilatation at the site of aneurysm produced a large perforation, which was sealed off immediately with a covered stent. Unfortunately, the patient had sudden cardiac tamponade and arrest later in the intensive cardiac care unit due to repeat perforation, and could not be resuscitated from this complication. Aneurysmal and ectatic arteries have fragile walls and aggressive post-dilatation for achieving optimal stent apposition should be avoided.
INTRODUCTION
Coronary artery aneurysm (CAA) is defined as coronary dilatation that exceeds the diameter of normal adjacent segments or the diameter of the widest coronary artery by a factor of 1.5.1 It differs from coronary artery ectasia where >50% of the length of the artery is dilated. The mean incidence of CAAs is 1.65%1 and the most common cause for CAA is atherosclerotic coronary artery disease (CAD), especially in adults. The common causes in children are vascular inflammatory diseases such as Kawasaki disease, systemic vasculitis, and congenital aneurysms.2 In the majority of cases, these aneurysms are identified incidentally during angiography procedures, but can also present with symptoms when they develop complications such as thrombosis followed by embolisation, vasospasm, and occasionally rupture. Management of CAA during acute coronary syndromes may represent a unique clinical challenge, as they have a high thrombus burden. Controversy surrounds whether conservative, surgical, or catheter-based management should be considered in these rare cases,1,2 as high thrombus burden, tortuosity, and significant spasms make intervention quite challenging and complex. Perforation, as a complication during intervention, has not been discussed before. Shimony et al.3 found, after meta-analysis of several studies, that the incidence of coronary artery perforations during percutaneous interventions was 0.43%.3 There are many causes for iatrogenic coronary perforations, depending on the lesion complexity.3 There are a few case reports of high-pressure,4,5 post-dilatation related coronary perforations in the literature, but none while intervening in ectatic or aneurysmal arteries. Moreover, recurrence of perforation after treatment in the catheterisation laboratory by covered stents has not been reported until now. We learned an important lesson during the initial case, and for subsequent cases we adopted a less aggressive approach while stenting aneurysmal or ectatic coronary arteries by avoiding high-pressure post-stent dilatations.
CASE REPORT
The first case was a 62-year-old hypertensive female, with no other cardiac risk factors, who presented to the emergency department following 2 days of intermittent angina at rest. An electrocardiogram showed inferolateral ST depressions with T wave inversions. An echocardiogram (ECHO) showed inferior wall hypokinesia with an ejection fraction of 54% and blood tests revealed elevated cardiac enzymes. The patient was diagnosed as having experienced a non-ST-segment elevation myocardial infarction, and initially given intravenous heparin, nitrates, and glycoprotein IIb/IIIa inhibitors. Later, due to persisting chest pain, an invasive strategy was planned. A coronary angiogram showed the mid-left anterior artery with 90% discrete short segment stenosis and large left circumflex artery (LCx) with proximal total thrombotic occlusion. Immediate percutaneous coronary intervention (PCI) of the culprit vessel was performed. The lesion was crossed with Runthrough® wire (Terumo, Tokyo, Japan) and thrombosuction was carried out with the Export® catheter (Medtronic, Fridley, Minnesota, USA). After the proximal vessel thrombosuction, some recanalisation was achieved and checkshot revealed a tight stenotic lesion, which required pre-dilatation with a Maverick™ 2.0×12 mm balloon (Boston Scientific, Marlborough, Massachusetts, USA). There was an aneurysmal segment in the LCx after the tight stenosis, and the entire lesion was covered with a 3.0×33 mm Promus Element™ stent (Boston Scientific, Marlborough, Massachusetts, USA), deployed at 12 atm. The stent at the aneurysmal region appeared malapposed, and hence post-dilated with 3.5×8.0 mm non-compliant balloon at 14–16 atm. To our dismay, the LCx artery developed a large Type III coronary perforation at the mid-portion of the stent, which was temporarily occluded with the stent balloon. Heparin was reversed with intravenous protamine and the surgeons were alerted of the complication. ECHO showed only mild pericardial effusion and, as the patient was haemodynamically stable, pericardiocentesis was not immediately performed. The perforation was sealed with 3.0×19 mm Graftmaster® covered stent (Abbott Vascular, Chicago, Illinois, USA) deployed within the previous stent at 12 atm by slow inflation (Figure 1). The patient was later transferred to the intensive cardiac care unit (ICCU) and was monitored for increased pericardial effusion or signs of stent thrombosis, as there were four layers of metal and no anticoagulation or antiplatelet drugs were administered. The patient remained haemodynamically stable overnight and serial ECHOs showed only minimal pericardial effusion with no evidence of tamponade, a similar profile to that observed at the end of the complicated PCI. Eighteen hours post-procedure, the patient developed sudden cardiac arrest in the ICCU and bedside ECHO showed large pericardial effusions with hypokinetic inferoposterior and lateral walls. Without delay, bedside pericardiocentesis was performed, immediately draining a significant amount of haemorrhagic fluid. Despite the maximum efforts, the patient’s vitals never recovered, and after unsuccessful cardiopulmonary resuscitation, the patient was declared dead in the ICCU.
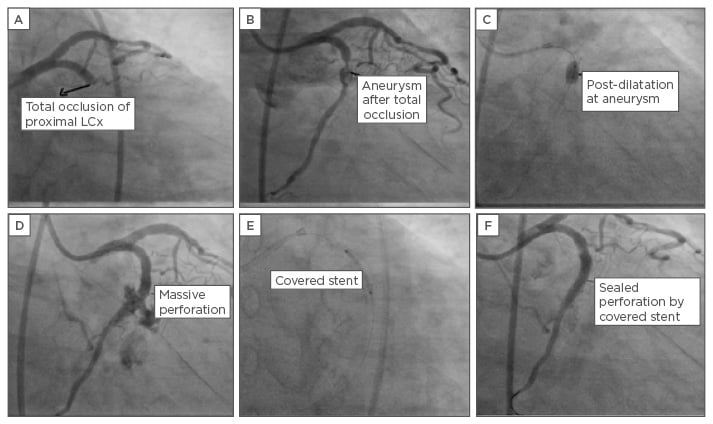
Figure 1: Coronary angiography during stenting procedure.
A) Total thrombotic occlusion of LCx; B) post-thrombosuction stenosis followed by aneurysm; C) post-dilatation of LCx; D) massive perforation at mid portion of stent; E) successful sealing of perforation by covered stent.
LCx: left circumflex artery.
The second case was a middle-aged female with an aneurysm in the LCx, and this time without pre or post-dilatation, we stented the aneurysmal lesion with 2.5×33 mm long bare-metal stent (BMS) ([Figure 2). This patient had a higher bleeding-risk profile, the reason for which a BMS was chosen. The patient is doing well after 1 year of follow-up.
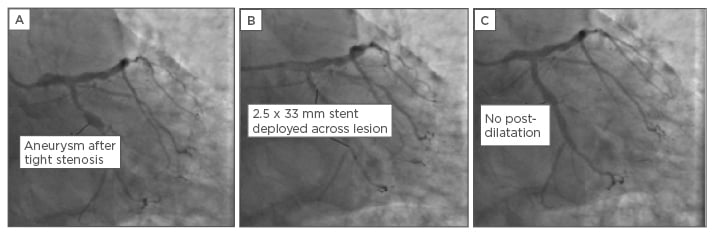
Figure 2: Coronary angiography images of bare-metal stenting of an aneurysmal lesion.
A) Stenotic lesion followed by aneurysm in LCx; B & C) lesion stented without post-dilatation.
LCx: left circumflex artery.
DISCUSSION
Based on the analysis of several angiography studies, the incidence of CAAs was found to range widely: 0.3–5.3%,1 and one of the reasons for the variability is the difficulty of distinguishing aneurysms from ectasia.2 CAA incidence is much higher in Indian patients than the general population, and they often have diffuse involvement of multiple arteries (10–12%).1,6 Aneurysms can be saccular (the transverse larger than the longitudinal axis) or fusiform (longitudinal at least twice the transverse axis),2 and the diameters of aneurysms range from 4.0–22 mm. CAAs and ectasia are variants of atherosclerotic disease and not a separate entity.2 The right coronary artery is the most commonly involved (40–87%) followed by the left anterior descending artery and LCx.1 Other CAA aetiologies include congenital, (accounting for 20–30% of cases and tending to be massive), inflammatory vasculitis, and connective tissue disorders.2 Iatrogenic aetiology following attempted PCI is rare but has been documented,7 resulting from residual dissection and deep-arterial wall injury following the use of oversized balloons or stents, high-pressure inflations, and atherectomy.
In adults, atherosclerotic disease is the most common cause of aneurysms, which tend to occur after obstructive lesions, such as the cases outlined above. Associated CAD is more severe in patients with discrete aneurysms than in those with diffuse ectasia. The combination of a proximal tight stenosis and a region of slower coronary blood flow immediately adjacent within an aneurysmal artery acts as a powerful nidus for thrombus formation. Additionally, turbulent post-stenotic flow within the coronary aneurysm promotes endothelial activation, attracting platelets and thrombin.
Histopathologic findings of autopsies have revealed extensive atherosclerotic changes with destruction or thinning of the tunica media of the vessel walls similar to that seen in atherosclerotic CAD.1 Matrix metalloproteinases have been implicated in the pathogenesis of CAA formation through increased proteolysis of extracellular matrix proteins.2 The heterogeneous depth of vascular injury caused by inflammation and atherosclerosis leads to varying degrees of remodelling within the coronary artery, whereby aneurysmal segments may lie adjacent to segments of stenosis. CAA occurs when the atheromatous process affects the intima, media, and adventitial parts of the vessel, leading to remodelling and dilatation. CAA then progresses according to Laplace’s law (increasing diameter leads to increasing wall stress), which in turn causes progressive dilatation of the affected arterial segment.2,8 In view of rare occurrence, clinical trials are not possible, only case reports or series are available, and treatment has to be individualised. It is generally opined that CAAs do not rupture unless extremely large.
If these lesions are detected incidentally during angiography, it is advisable to manage medically, rather than surgically, due to uncertain procedural outcomes. Some advocate chronic anticoagulation to prevent thrombosis of aneurysmal arteries, but sometimes despite anticoagulation, patients develop thrombotic occlusion, suggesting that other mechanisms, such as plaque rupture, are involved in the development of acute coronary, in this context.1 If patients present with acute coronary syndromes, they are treated with antiplatelets, nitrates, thrombolysis, or glycoprotein IIb/IIIa inhibitors. Either BMS or drug-eluting stents (DES) may be considered with PCI of aneurysmal arteries, but stent malposition is potentially problematic and may lead to stent thrombosis, restenosis, or thrombus embolisation producing slow or no reflow. Some authors suggest using polytetrafluorethylene (PTFE)-covered stents as a better method to trap thrombi and exclude aneurysmal portions of coronary arteries, but this presents a risk of branch-vessel occlusion and high incidence of restenosis.9 PTFE-covered stents should be used when the aneurysm diameter is <10 mm, but surgery is preferable for larger diameter aneurysms.1 As there are no guidelines available, the optimal approach is decided on a case-by-case basis depending on the technical expertise available, patient factors, and lesion characteristics. For example, if the vessels were heavily calcified or tortuous, PTFE-covered stent delivery would be difficult, and if the lesion is very long (as in the above cases), the correct size of covered stent may not be available, especially in emergency situations.2 However, if they are successfully delivered, they can theoretically seal the aneurysm, thereby preventing the risk of rupture or distal embolisation of debris. If PTFE-covered stents cannot be used due to their various disadvantages, DES or BMS must be considered. While initial reports suggested that implantation of first-generation DES led to higher incidence of associated late CAA compared with BMS (1.4% versus 0.2%), there are limited data on the current generation of DES.10 Other indications for surgery in CAA treatment are involvement of the left main coronary artery, associated multi-vessel CAD, and bifurcation of significant side-branch vessels.2 The most common treatment is surgery, especially in adults, according to a coronary artery surgery study (CASS) sub-study, which concluded that surgery in CAA patients had comparable results to routine coronary artery bypass grafting.11 In both of the aforementioned cases, surgery was not primarily considered due to isolated circumflex artery involvement. Use of self-expanding stents is another treatment option with PCI of these arteries. Regardless, due to the high propensity of developing thrombosis and embolisation, dual antiplatelet therapy must be continued for a long duration after artery stenting. Aspirin and oral anticoagulants may be used to maintain the international normalised ratio in the 2.0–2.5 range if thrombosis recurs on dual antiplatelet therapy. The role of novel oral anticoagulants is not clear in these types of cases.
Important risk factors for coronary perforations can be divided into patient-related factors and angiographic characteristics of the lesions. Patient-related risk factors include: older age, female sex, hypertension, history of heart failure, renal failure, and prior use of antithrombotics. Angiographic factors include coronary calcification, chronic total occlusions, lesions >10 mm in length, tortuous lesions, Type C lesions, use of hardware such as hydrophilic or stiff wires, atherectomy devices, intracoronary ultrasounds, and post-dilatation of stents at very high pressures. Aneurysmal coronary arteries and ectatic arteries were not mentioned as risk factors for coronary perforation during interventions.8,12 There are different methods of treating coronary perforations, ranging from a simple reversal of anticoagulation in the case of guidewire-related perforations, to emergency surgery in the case of large perforations, but the use of PTFE-covered stents has emerged as the standard treatment.13 The main concerns regarding the use of PTFE-covered stents in perforation setting are occurrence of stent thrombosis and in-stent restenosis. Initial studies have reported rates of 33% for stent thrombosis, and 50% for in-stent restenosis at 6 months, due to delayed stent endothelisation with the PTFE membrane.14
Sealing perforations with a covered stent may be unsuccessful, as the entire vessel wall may be diseased, leading to the eventual giving-way of adjacent areas, and reperforation. As a result of the previously mentioned case, we dealt cautiously with ectatic or aneurysmal artery PCI, avoiding high-pressure post-stent dilatations at aneurysm sites. Gaps between unopposed stents and aneurysmal walls are sealed in due course, and routine antiplatelets with a short course of anticoagulation suffice as treatment.
CONCLUSIONS
CAAs are risk factors for coronary perforation, and high-pressure post-stent dilatation to treat stent malapposition at aneurysmal sites should be avoided.








