Abstract
A 61-year-old male with chronic cough, paraesthesia of the extremities, and sinusitis presented for acute worsening of symptoms despite initial treatment with antibiotics and prednisone. Emergency department evaluation revealed mild elevated troponin without ECG changes in absence of coronary symptoms, but markedly elevated eosinophil count and an abnormal chest CT. A nuclear stress test revealed basal and inferoseptal dyskinesis with fixed apical defect. Left heart catheterisation revealed multiple coronary stenosis requiring intervention. Further extensive work-up confirmed a diagnosis of hypereosinophilic syndrome complicated with respiratory, cardiac, gastroenterological, and neurological involvement. The patient was initially treated with a high dose of intravenous steroid and hydroxyurea. Flow cytometry revealed negative FIP1L1-PDGFRA gene rearrangement, but was positive for JAK2 V617F mutation and perinuclear antineutrophil cytoplasmic antibodies/cytoplasmic antineutrophil cytoplasmic antibodies, indicating possible overlap of eosinophilic granulomatosis with polyangiitis.
Key Points
1. Hypereosinophilic syndrome (HES) describes a large group of disorders characterised by overproduction of eosinophils that typically infiltrate and cause damage to major organs. Only a few articles report HES in association with JAK2 V617F mutation, and typically this is in conjunction withchronic myeloproliferative neoplasm.
2. This unique case of HES with JAK2 V617F mutation illustrates that HES, when combined with either essential thrombocytosis or other hypercoagulable states, can lead to multi-organ involvement, including coronary artery thrombosis and stenosis.
3. Routine screening ECGs are recommended in patients with HES for evidence of acute thrombosis formation in the absence of acute cardiac symptoms. With essential thrombocytosis, antiplatelet therapy and, in more severe cases, anticoagulation therapy could be beneficial for acute coronary disease
prevention; further meta-analysis is necessary for understanding clinical outcomes.
INTRODUCTION
Hypereosinophilic syndrome (HES) refers to a large group of disorders characterised by overproduction of eosinophils that typically infiltrate and cause damage to major organs. HES can be subclassified by the mechanism that leads to eosinophilic elevation. Primary HES typically occurs in the setting of a neoplastic or myeloproliferative disorder. Secondary HES is reactive and polyclonal, typically lymphocytic. Idiopathic and specific syndromes can additionally be associated with HES, such as eosinophilic granulomatosis with polyangiitis.1 In neoplastic or myeloproliferative disorders, the most common molecular mutations are tyrosine kinase fusion genes with involvement of PDGFRA, such as FIP1L1-PDGFRA fusion. Point mutations such as JAK2 V617F constitute a relatively small proportion of HES, and are typically associated with the subtype of myeloproliferative HES.2
Cardiac involvement of HES is not very common as the disease itself is rare. Ogbogu et al.3 described the staged damage of myocardium based on the chronicity of disease. Ogbogu et al.3 observed acute myocardial necrosis within a mean time of 5.5 weeks from initial diagnosis, whereas thrombus formation was noted within 10 months and fibrosis at the 24.5 month mark.4,5 Cardiomyopathy is theorised to be secondary to ventricular vascular damage, but subvalvular involvement has also been reported. Echocardiogram, cardiac MRI, and endomyocardial biopsy remains the mainstay of diagnosis, and treatments are usually disease-directed conventional therapy. This manuscript reports a unique case of HES in addition to JAK2 V617F-mediated essential thrombocytosis with multi-organ involvement. Most importantly, the authors report a case of myocardial infarction in the absence of acute cardiac symptoms.
CASE PRESENTATION
A 61-year-old White male presented to the emergency department complaining of chronic cough. The patient stated that the cough had begun 5 months prior and that they had been intermittently placed on azithromycin and prednisone, with the most recent course ending 1.5 weeks prior to their presentation. The patient endorsed a productive cough with white sputum, night sweats, a 5 lb weight loss, and decreased appetite over the course of the previous week. They also noted fatigue and bilateral upper and lower extremity (‘glove-and-stocking’) paraesthesia in the previous few days. The patient denied chest pain or shortness of breath with no fevers or chills. The patient was tested for COVID-19 a few days before their visit to the emergency department, for which they presented a negative result.
Pertinent medical history included asthma and an unprovoked deep vein thrombosis in 2019. Social history showed significant tobacco use for 5 years, with the patient quitting 10+ years ago.
Upon arrival to the emergency department, the following vitals were obtained: blood pressure of 138/87; pulse of 83 beats per minute; temperature of 36.6 °C; respiratory rate of 20 breaths per minute; and oxygen saturation of 94%. A physical exam was significant for subjective paraesthesia in hands and legs bilaterally upon neurological testing, but the remaining examination was otherwise benign. Initial lab testing included a complete blood count, which was significant for the following: white blood cell count of 25.5; platelet count of 523; absolute eosinophil count of 9.4; erythrocyte sedimentation rate elevated at 46; and troponin of 0.790. Initial diagnostic testing included ECG interpreted as sinus rhythm with a rate of 85 beats per minute, and no evidence of acute infarct. CT angiogram of the chest demonstrated patent pulmonary arteries with no central filling defect, subtle ‘tree-in-bud’ opacities, mild peribronchial thickening in both lung bases, and hilar lymphadenopathy. CT angiogram additionally demonstrated a small right-sided pleural effusion.
Initial differential diagnoses included atypical pneumonia, vasculitis, pneumoconiosis, allergies, fungal and parasite infection, collagen vascular disease, HES, and potential drug side-effects.
Upon further questioning, the patient reported a chronic intermittent cough for the past 2 years, with a chronic history of sinusitis. They also noted owning a bird feeder that they changed regularly, and further stated that they had been planting trees in the fields behind their house for the past couple of weeks. Lastly, they reported new-onset dysphagia to food and wheezing. The patient was heterosexually monogamous with their wife.
It was decided to consult infectious diseases, pulmonology, and cardiology. Due to the patient’s CT angiogram and previous lab results, HIV labs, antinuclear antibody, and an Ig panel were drawn, all of which were within normal limits. Folate and B12 levels in relation to the new-onset stocking-glove paraesthesia were insignificant. Due to the patient’s elevated troponin levels, a heparin drip was ordered, while maximum troponin (1.7) was continuously monitored with serial ECG. An inflammatory panel revealed elevated C-reactive protein as well as a total creatine kinase of 626.
Pulmonology evaluated the patient and recommended a fungal/parasite panel, which was negative. Blood cultures showed no growth over 48 hours.
Cardiology believed the presentation could possibly be attributed to viral myocarditis and ordered the following panels: cytomegalovirus, Epstein–Barr virus, Coxsackie B, hepatitis C, parvovirus, and mycoplasma. All showed levels that were within normal limits. Cardiac lipid profile was additionally insignificant (Table 1). An echocardiogram without contrast was performed and showed no focal wall motion abnormalities; estimated ejection fraction of 55%; normal right ventricular size and function; and no left ventricular thrombus. Nuclear stress imaging demonstrated a reduced left ventricular ejection fraction of 44% with a fixed defect in the apical wall and dyskinesis of the basal inferoseptal segment (Supplementary Figure 1). At this point, the patient continued to deny experiencing any chest pain. Following the abnormal stress test, a left heart catheterisation with selective coronary angiography was performed that revealed mildly reduced left ventricular systolic function with ejection fraction of 45%. A moderate coronary atherosclerosis of 50% in the posterior lateral branch was identified. Upon second review of the study by a different cardiologist, it was deemed that the patient’s posterior coronary artery was potentially severe rather than moderate, and a repeated left heart catheterisation was planned. The patient remained free of chest pain and haemodynamically stable. On Day 4, a second coronary angiography with contrast showed that the mid-left anterior descending vessel had a focal stenosis of 80%, with evidence of post-stenotic dilation. Instantaneous wave-free ratio was performed for functional testing and resulted in an abnormal value of 0.74, indicating ischaemia. Therefore, a drug-eluting stent was placed in the mid-left anterior descending vessel. The posterior left ventricular branch appeared to show moderate-to-severe stenosis up to 70%, but presented as normal on instantaneous waveform ratio, and was left for medical therapy (Supplementary Figure 2).
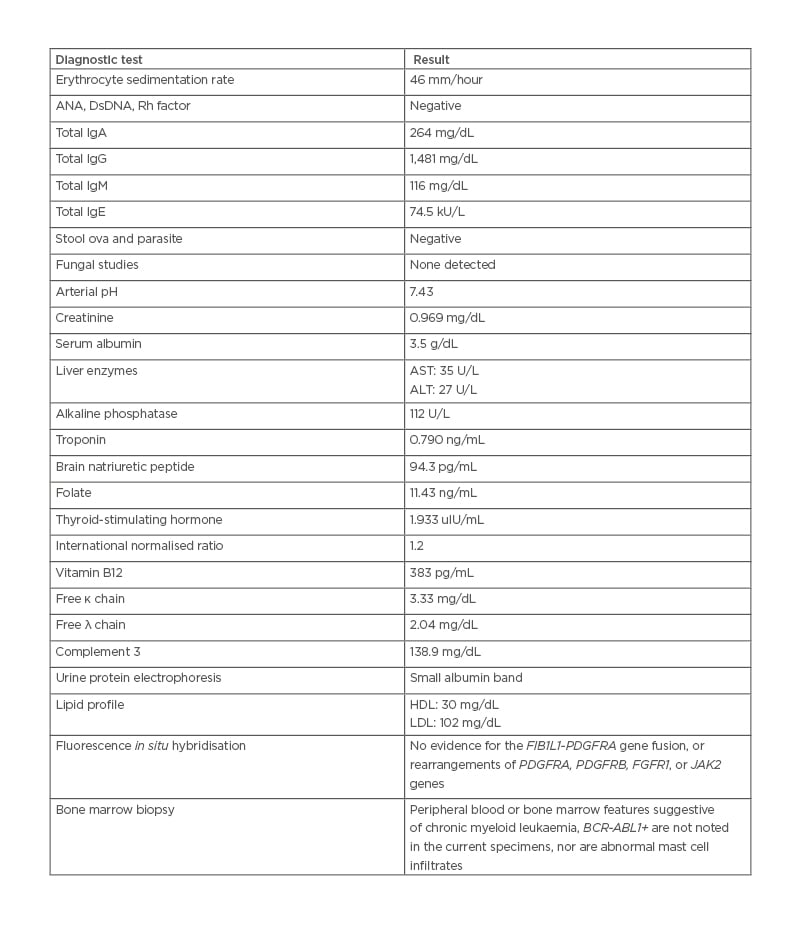
Table 1: Summary of pertinent diagnostic test for new diagnosis of hypereosinophilic syndrome and essential thrombocytosis.
ANA: antinuclear antibody; ALT: alanine transaminase; AST: aspartate transaminase; DsDNA: double-stranded DNA antibody; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Rh: rheumatoid.
Despite elevated eosinophil levels, as the fungal, parasite, and viral panels were negative, and the patient also exhibited extensive cardiopulmonary involvement, perinuclear antineutrophil cytoplasmic antibodies and cytoplasmic antineutrophil cytoplasmic antibodies were additionally obtained. These showed elevated titres of 1:320 with a positive myeloperoxidase antibody index. Eosinophil levels continued to increase from 9.4 K/μL at admission to 11.1 K/μL on Day 3. Haematology and oncology were consulted as the combination of symptoms presented were concerning for potential HES. BCR-ABL1 and FIP1L1-PDGFRA, as well as flow cytometry of the patient’s peripheral blood smear, were further obtained. Figure 1 illustrates blood smear findings that revealed leukocytosis reflecting marked eosinophilia (absolute eosinophil count approximately 11,000/μL), but no features suggestive of acute leukaemia. Fluorescence in situ hybridisation was negative for BCR-ABL and FIP1L1-PDGFRA. JAK2 testing was added due to the patient’s platelet count, which continued to trend upward from admission, and was found to be positive for JAK2 V617F mutation. A bone marrow biopsy was obtained, which showed modest hypercellular marrow, as well as increased maturing eosinophils and mature granulocytes. Serum protein electrophoresis was remarkable for a polyclonal increase in IgG4. Urine protein plasmapheresis was negative for Bence Jones proteins.
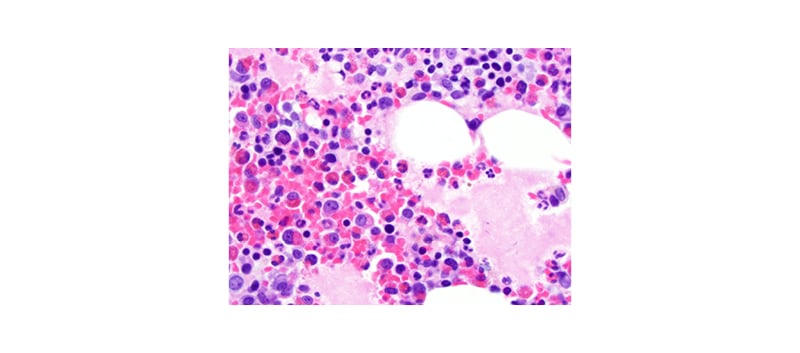
Figure 1: Core biopsy confirming infiltration of eosinophils in the bone marrow correlated with peripheral finding of eosinophilia.
Varas-Lorenzo et al.6 demonstrated that use of oral corticosteroid therapy of more than 10 mg/day for 30 days increases risk of acute myocardial infarction. Since immunosuppressive benefits of steroids in the setting of acute hypereosinophilic syndrome outweighs relative increased risk of myocardial infarction, the patient was started on intravenous Solu-Medrol (Pfizer Inc., New York City, New York, USA) 125 mg daily. The patient subsequently reported feeling symptomatically better after starting steroids and showed mildly improved eosinophilia levels.
Following this, the patient was officially diagnosed with hypereosinophilia of unknown aetiology with plans to be discharged on Day 5 of admission, with prescription for prednisone 60 mg daily and an outpatient follow-up referral with haematology and oncology. The patient was also diagnosed with acute myocardial infarction and cardiac findings were suspected to be secondary to myocarditis with hypereosinophilia. The patient was started on dual antiplatelet therapy with aspirin and prasugrel, as well as a statin, angiotensin-converting enzyme inhibitor, and β-blocker.
On follow-up with haematology and oncology, it was suspected that the patient had an overlap with hypereosinophilic syndrome, as well as JAK2+ essential thrombocytosis after examination of the patient’s lab work. Figure 2 demonstrates the course of eosinophil response with immunosuppression therapy over time. The patient’s initial complaints of cough, dysphagia, and generalised fatigue were resolved shortly with treatment. Initiation of hydroxyurea maintained long-term suppression, and no further eosinophilia or deteriorating symptoms were noted over the course of 12 months.
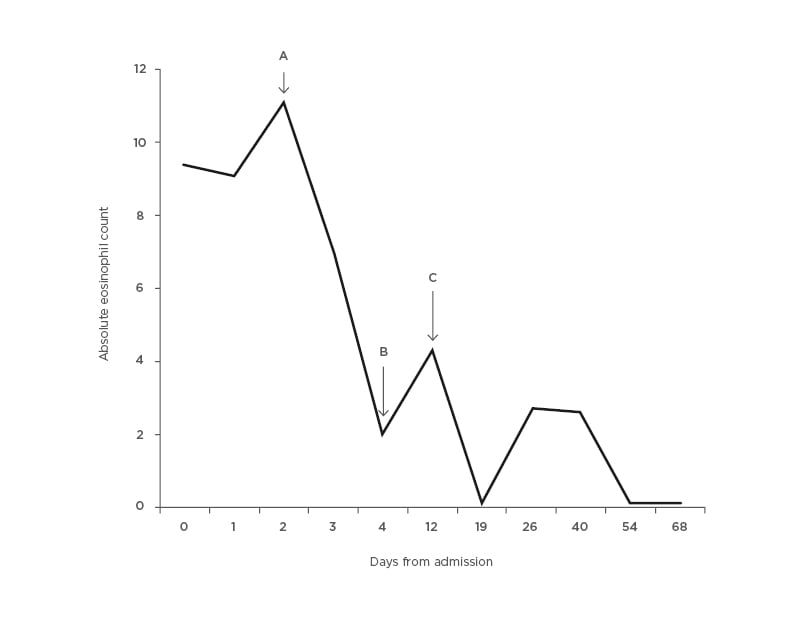
Figure 2: Absolute eosinophilic count trended with time and response to therapy.
Initiation of immunosuppression with steroid therapy significantly suppresses eosinophil count and improves clinical symptoms. Addition of hydroxyurea maintains long term suppression. A) Intravenous Solu-Medrol (Pfizer Inc., New York City, New York, USA) 125 mg started. B) Discharged from hospital (on prednisone 60 mg). C) Prednisone increased to 80 mg and hydroxyurea 500 mg daily added.
The patient additionally visited rheumatology, who were unable to perform a biopsy to assess eosinophilic granulomatosis with polyangiitis as the patient recently received high-dose steroids. Based on the lab findings and clinical involvement of the heart and other organs, the patient is currently being evaluated for possible monthly intravenous cyclophosphamide infusion instead of hydroxyurea in combination with steroids.
DISCUSSION
HES is a group of disorders with markedly elevated eosinophils as well as tissue infiltration. It is defined as a persistent absolute eosinophil count >1,500 cells/μL in peripheral blood on at least two examinations with the presence of eosinophil-mediated organ damage.7 There are a variety of clinical variants of HES categorised by their causes: myeloproliferative, lymphocytic-associated HES, familial, idiopathic, and specific syndromes associated with HE (overlap HES).8 Based on previous studies and reports, venous thrombosis is a well-known complication.7,8 It is interesting that many of these studies report venous thrombosis in regards to Budd–Chiari manifestation,7 whereas this patient’s possible major manifestation was in an unprovoked deep vein thrombosis 1 year prior.
Only a few articles report HES in association with JAK2 V617F mutation, and typically this is in conjunction with chronic myeloproliferative neoplasm.7,9 Furthermore, it was found that in documented cases with positive JAK2 V617F and a normal karyotype lacking FIP1L1-PDGFRA, patients were more resistant to standard treatment with steroids and hydroxyurea.9 Interestingly, this patient seemed to improve with these therapies. Peripheral neuropathy accounts for a majority of the neurologic manifestations of HES; however, the pathophysiology remains largely unexplained and should be further investigated.10
With multi-organ infiltration, it is well known that elevated eosinophils can cause damage to the heart, and eosinophilic myocarditis is a major cause of morbidity and mortality among patients. Loeffler’s endocarditis is another manifestation of HES, described as eosinophil degranulation causing specific toxic effects of cationic protein on the plasma membrane leading to endomyocardial disease.11 Echocardiogram and CT imaging are initially performed to assess for left or right ventricular apical obliteration or thrombi formation, as well as evidence of restrictive cardiomyopathy. Endomyocardial biopsy remains the gold standard to diagnose Loeffler’s endocarditis.12 Interestingly, no apical obliteration, left ventricular thrombus, or clinical manifestation of restrictive cardiomyopathy were noted in this patient. Retrospectively, echocardiogram with contrast, and/or cardiac MRI with possible, if indicated, endomyocardial biopsy should have been performed to rule out atypical Loeffler’s endocarditis or endomyocardial fibrosis. Further, an intracoronary imaging would have been beneficial to characterise the morphology of atherosclerotic plaques, erosion, or rupture.
In relation to multivessel coronary stenosis during eosinophilic crisis, the aetiology of sudden myocardial infarction remains unknown. Due to their advanced age, gender, mild dyslipidaemia, and smoking history, this patient is susceptible to above-average cardiovascular risk for plaque rupture. However, their troponin elevation during eosinophilic crisis raises the question as to whether coronary stenosis was acute precipitation of exaggerated plaque rupture and thrombosis due to hypercoagulable state from HES, or essential thrombocytosis, or a combination of both. Individually, it is hypothesised that toxicity from excessive eosinophils and the clotting cascade activation is one of the mechanisms of myocardial injury in HES. In the presence of JAK2 V617F, the plethora of platelets perhaps further propagates thrombosis formation at a much faster rate than either disease separately. If thrombosis is the aetiology, this can explain why acute myocardial infarction and stenosis precipitated shortly after the onset of eosinophilic syndrome. In addition to acute thrombus formation, myocarditis, endomyocardial fibrosis, and atypical Loeffler’s endocarditis need to be considered in differential diagnosis. Further basic science research in vivo models can decipher signalling pathways, and likely identify key players to target for hypercoagulable state in this overlapping syndrome.
In summary, this case report describes a variant of overlapping HES with JAK2 V617F mutation presenting with asymptomatic myocardial injury, peripheral neuropathy, eosinophilic oesophagitis, pulmonary symptoms, and remote deep vein thrombosis with adequate response to corticosteroid therapy and hydroxyurea. This case illustrates HES, when combined with either essential thrombocytosis or other hypercoagulable states, lead to multiple organ involvement, especially coronary artery stenosis due to potential thrombosis. Appropriately treated patients are at increased risk for opportunistic infection and immunological complications. Table 1 summarises an extensive list of workups for new diagnosis of HES, and the respective results. Therefore, it is recommended to check ECGs routinely to screen for acute thrombosis formation in absence of myocardial injury, pericardial injury, and valvular involvement, and shows necessity to optimise modifiable cardiac disease risk factors in patients with HES despite age, gender, and ethnicity. When presented in the emergency department, regardless of cardiac symptoms, ECG and cardiac enzymes should be checked with high suspicion for myocardial infarction secondary to acute thrombosis and accelerated plaque rupture. If admitted to hospital, an echocardiogram should be performed, checking for subvalvular involvement, new wall motion abnormalities, and fibrosis evaluation. Follow-up during discharge should be monitored closely with cardiology, haematology, and rheumatology. With essential thrombocytosis, antiplatelet therapy, and, in more severe cases, anticoagulation therapy would be beneficial for acute coronary disease prevention. Further meta-analysis is necessary for understanding clinical outcomes.







