Abstract
Chagas disease is a tropical illness characterised by arrhythmias, heart failure, and eventually death. In approximately 10–30% of patients, chronic disease appears 10–30 years after infection onset. One of the biggest challenges for treatment is how to manage disease progression during the non-symptomatic phase to avoid the most life-threatening consequences of Chagas disease. The aim of this review is to evaluate the empirical rationale for an alternative therapy based on pathophysiological mechanisms that lead to chronic cardiac pathology and that have the possibility of evaluation through serological markers. The author identifies L-arginine serum levels, IL-2, and short-form Cha autoantibodies as possible markers for Chagas disease and discusses the reports regarding the therapeutic potential of amiodarone and angiotensin-converting enzyme inhibitors to modulate the electrophysiological, inflammatory, and vascular disturbances that lead to symptomatic Chagas disease. This review considers this discussion to improve the comprehension of therapeutic alternatives based on the vast literature detailing Chagas disease’s pathophysiology.
INTRODUCTION
Chronic Chagasic cardiomyopathy (CCC) is one of the principal challenges for clinicians treating asymptomatic Chagasic patients. Only benznidazole (BZ), a DNA destabiliser, is approved by the U.S. Food and Drug Administration (FDA) for Chagas disease treatment. The drug is most prominently used in Europe for the treatment of Latin-American immigrants infected with Trypanosoma cruzi. There is controversy regarding the treatment of chronic phase Chagasic patients, especially considering the potential side effects associated with BZ, including granulocytopenia, rash, and digestive alterations. Additionally, in a large trial published in 2015, BZ treatment did not show a significant improvement in the outcome of Chagas disease in chronic patients.1 Similarly, combination therapy using posaconazole and BZ in another study had no effect.2 A study by Sguassero et al.3 has suggested a long-term trypanocidal effect after BZ treatment but with an imprecise relationship with improvement of clinical condition.
Alternatively, non-trypanocidal therapy, implemented to avoid the symptomatic and structural alterations associated with Chagas disease, has been suggested as a possible factor that helps control Chagasic cardiomyopathy. In this case, amiodarone, a drug used to control malignant arrhythmias in Chagas disease, was associated with the best clinical outcome when combined with BZ in the BENEFIT trial.1 This represented an interesting finding due to the previously reported trypanocidal potential of amiodarone4 and the possible immunomodulatory effects of these drugs.5 In experimental models, dipyridamole (coronary vasodilator agent) and captopril (angiotensin- converting enzyme [ACE] antihypertensive agent), among others, have been associated with an amelioration of Chagasic cardiomyopathy,6,7 which suggests their use together with trypanocidal therapy to palliate the inflammation induced by T. cruzi and thereby obtain clinical improvement of Chagasic patients, especially before the establishment of cardiac pathology.
This review will analyse the pathophysiological causes associated with trypanocidal effects and improvement of clinical outcome in chronic patients. In addition, an approach using adjuvant therapies that could be implemented in the early phases of infection will be proposed. Finally, the authors highlight the necessity of more clinical trials to evaluate treatment schemes for patients with concomitant cardiovascular pathology.
CHRONIC CHAGASIC CARDIOMYOPATHY: THE HOLY GRAIL OF RESEARCH IN THERAPEUTICS
Chagas disease progresses to the chronic phase in approximately one-third of infected patients; this is often 10–30 years after the first infection. CCC is the most severe consequence of chronic infection and has a deep economic impact on the healthcare system. For instance, lifetime costs per patient have been calculated at $11,619.00 per patient in Colombia8 and global costs have been estimated at \$7.19 billion per year and $188.80 billion across the total lifetime of all patients.9 Additionally, the same study calculated the disability-adjusted life-years to be 3.57 years for Chagasic patients, which suggests a considerable loss in quality of life and work capacity as a result of this infection. Therefore, it is important to design a rational approach that allows for the avoidance of CCC development. However, this design supposes comprehension of the pathophysiological determinants of evolution to CCC, which is a goal far from being reached.
CCC is a complex condition that involves an unbalanced inflammatory process which impacts cardiac remodelling, electrical conduction, and vascular integrity, which, in turn, leads to a complex cardiomyopathy with mixed pathophysiological mechanisms normally associated with different cardiomyopathies (dilated, ischaemic, and/or hypertensive). Macroscopically, CCC is characterised by thickening of the ventricular walls and cardiomegaly, the development of small nodules around the epicardium, and intracavitary thrombus.10 Furthermore, Suárez10 classified the progression of CCC into four phases: normal, ambiguous, type 1, and type 2. The type 2 stage is characterised by global chamber dilatation, cavitary thrombosis with coronary sclerosis, endocardial engrossment, and apical aneurism; patients with type 2 phase CCC often die as a result of of heart failure and/or malignant arrhythmias.
The pathophysiological mechanisms driving CCC may be divided into four categories: vascular, autoimmune, oxidative, and parasite-derived.
Vascular Involvement in Chronic Chagasic Cardiomyopathy Development
The role of vasculature in Chagas disease pathology has been documented since the 1980s. Early studies reported myocardial vascular occlusion in experimental models of chronic Chagas disease.11 Later studies demonstrated sub-epicardial and endocardial ischaemic foci,12 coronary circulation disturbances with areas of focal vascular constriction, microaneurysm formation, vessel dilatation, and proliferation of microvessels.13 Other work has associated endothelial infection with the activation of the NFκB pathway and the upregulation of vascular cell adhesion molecule 1 and E-selectin.14 Additionally, endothelin 1, a potent vasoconstrictor, was secreted during parasite infection. The protein was identified in the supernatants of T. cruzi-infected human umbilical vein endothelial cells.15 Furthermore, COX-2, thromboxane synthase, inducible nitric oxide (NO) synthase, the p65 NFκB subunit, serum TNF-α, and p22 of NAD(P)H oxidase subunit expressions were increased in the vessels of Chagasic animals.16 Moreover, T. cruzi promotes a systemic increase in TNF-α, which stimulates inducible NO synthase expression in vessels promoting nitrosative stress16 and the production of IL-6 and TNF-α, which was evident in the inflammatory infiltrate in the endothelial and smooth muscle layers.17
Pathological changes are reflected in Chagasic patients’ outcome. Vascular disorders, especially thromboembolism, have been reported for >60 years.18 More recently, Chagasic cardiomyopathy has been associated with increased risk of ischaemic stroke.19 CCC is often under-considered in the clinical practice, even though the prevalence of apical aneurysm and mural thrombus in Chagas disease-related stroke patients has been estimated at 37.0% and 11.7%, respectively.20 However, stroke may be the initial manifestation of Chagas disease for many patients. Additionally, microcirculation abnormalities have been described in patients with normal coronary images. Thinning of the ventricular wall in CCC patients is related to ischaemic lesions in the peripheral territory irrigated by the right coronary artery.21 Studies of Chagasic patients with angiographically normal coronary arteries revealed myocardial perfusion abnormalities, which are thought to be associated with microcirculatory disturbances.22 More recently, impairment in coronary reserve was described in non-symptomatic Chagasic patients.23 Finally, endothelial adhesion markers have been investigated for an association with CCC. Increased levels of soluble platelet- selectin, a well-known endothelial marker,24 have been reported in CCC, reinforcing the idea that vascular dysfunction plays a key role in the development of chronic cardiomyopathy.
Oxidative Alterations in Chagasic Hearts
Mitochondrial Damage and Hypoxia
Oxidative reactions are well known mediators of cardiac disturbances during CCC. Beyond the fact that inflammation generates reactive oxygen species, the impact of infection itself regarding ischaemia and the generation of oxidative damage in cardiac cells and the possible impact of this phenomena in the evolution of CCC is further outlined below.
One of the most important organelles in cardiac functioning are the mitochondria. Different respiratory chain complexes have demonstrated increased activity in skeletal muscle mitochondria during chronic experimental infection.25 In cardiac cells of a CCC experimental model, increases in citrate synthase activity and changes in mitochondrial structure were detected.26 Another study has shown that there are subpatent mitochondrial changes in the evolution of indeterminate to symptomatic phase.27 Additionally, T. cruzi invasion elicits Ca2+ overload, mitochondrial membrane electrical potential transition,28 and a decline in the oxidative phosphorylation ability of the myocardium in a chronically infected mouse model.29
Mitochondrial dysfunction is closely linked to heart failure. Different pathophysiological mechanisms are associated with cardiac cell disturbances during heart failure. Intrinsic apoptotic pathways, reactive oxygen species-induced cellular damage, intracellular acidosis for anaerobic glycolysis, and ATP deficiency associated with contractility dysfunctions have been proposed as pathophysiological explanations of mitochondrial involvement in heart disease.30 Ongoing research should shed light on the possible cause and effect mechanisms involved in Chagas disease.
Autoimmunity in Chagas Disease
Independently of mechanisms involved in parasite persistence, the chronic stimulation of the immune system generates a proinflammatory state that predisposes to autoimmunity. Over 30 years ago, the link between autoimmunity and chronic Chagas disease, especially to arrhythmias, was made. This raised the question of whether the release of intracellular autoantigens during myocytolysis originating from a parasite invasion might generate an autoimmune response and produce cardiac dysfunction. Humoral autoimmune response has been associated with cardiac pathology in chronic Chagas disease.30 β1-adrenergic autoimmune responses have been associated with T. cruzi ribosomal P0 cross-reactivity31 and autoantibodies were shown to bind to the extracellular loop of this receptor and modulate calcium ion channels in cardiac cells32 and have since been associated with Chagasic cardiomyopathy progression.33 Moreover, antimuscarinic autoantibodies have been implicated in allosteric positive regulation of heart muscarinic 2 receptors.34 This modulation altered the vagal nerve function in Chagasic patients,35 which was postulated as a treatment response marker in children with Chagas disease,36 although the relationship of these antibodies to ventricular dysfunction is controversial.37 Additionally, myosin,36,38 galectin-1,39 cardiolipin,40 and troponin T41 have been reported in chronic Chagas disease, reflecting the complexity of the autoimmune response and the necessity of discriminating the role of each one in the pathology.
On the other hand, cytotoxic autoimmunity may have a role in chronic Chagasic cardiomyopathy. Autoreactive T cells, isolated from chronically infected mice, may generate unspecific alterations in heart repolarisation, cardiac inflammatory infiltration, and tissue damage when transferred to uninfected mice.42 T. cruzi antigens, such as B13, cruzipain, and Cha, cross-react with host antigens at the B or T cell level.43 These autoreactive cells can produce IFNγ and TNF-α; this characteristic may be linked to T. cruzi-induced IL-12 production.31 Inflammation linked to IL-2 response, a powerful T cell mitogen, has been implicated in the genesis of malignant arrhythmias and postulated as a prognostic factor and as a possible drug marker,5 relating for the first time in a direct way to arrhythmias and inflammation in Chagas disease. This array of autoimmune responses, in addition to proarrhythmogenic parasite secretory factors,44 microvascular angiopathy in the brain45 and in coronary circulation,46 continuous parasite invasion,47 and chronic oxidative damage to heart tissue,48 may generate chronic cardiac dysfunction, which leads to heart failure as well as valvular and hypertrophic cardiac pathology.
Parasite Involvement in Cardiac Electrical Disturbances and Remodelling
It is well known that T. cruzi secretes and sheds a wide variety of glycoproteins during cellular invasion and the differences among strain secretomes have been suggested to correlate with virulence.49 Previous work by the authors described that immunogenic proteins obtained from the secretome of T. cruzi, mainly of high molecular weight, were able to induce arrhythmias in an ex vivo isolated beating heart.50 Calcium overload associated with parasite invasion51 is one of the most plausible ionic mechanisms that explains the increasing action potential duration reported in cardiac cells infected with T. cruzi.52 Additionally, preliminary results recently published by the authors have shown evidence of overexpression of hydrogen cyanide 1 and 4 channels in the atria and ventricles of mice during acute infection.53
On the other hand, since the early 2000s, T. cruzi persistence in cardiac tissue from endomyocardial biopsies have been linked to inflammation, necrosis, and fibrosis. T. cruzi infection in a three-dimensional cardiac culture model induced fibrosis through activation of the TGF-β pathway.54 Additionally, genes associated with the immune response, inflammation, cytoskeleton organisation, cell-to-cell and cell-to-matrix interactions, apoptosis, cell cycle, and oxidative stress were among those affected during the infection of cardiac cells by T. cruzi.55 Taken together, these results suggest that parasite persistence may contribute to the pathophysiology of fibrosis and inflammation in chronically infected mice.
Neurohumoral and Autonomic Pathogenic Mechanisms
Chagas disease pathophysiology is a complex mix of mechanisms that lead to severe consequences in a portion of chronically diseased patients. One of the oldest theories regarding the generation of CCC is the imbalance of the autonomic nervous system.56 In the following years, parasitism in the sympathetic ganglionic chain was described in experimental models,57 which was associated with heart rate dysregulation in Chagasic patients58 and autoimmunity directed against neuronal tissue.59 More recently, control of orthosympathetic dysfunction has been linked to the improvement of arrhythmogenesis60 and chronic heart remodelling.61
A RATIONAL APPROACH FOR THE TREATMENT OF CHRONIC CHAGASIC CARDIOMYOPATHY
One of the principal challenges facing physicians treating patients with Chagasic disease is how to explain the diagnosis to the patient, which is especially difficult if they are not symptomatic. With a 10–30% probability of sudden death or developing heart failure over the long-term, toxicity is an important factor to consider when analysing the efficacy of conventional therapies.
Prognosis Factors in Chronic Chagasic Cardiomyopathy
One of the most challenging issues for the treatment of CCC patients is discriminating which patients are at risk of developing heart failure and/or dying suddenly and which are not. Several authors have contributed to the identification of markers that are associated with clinical outcome. Left ventricular systolic dysfunction and non-sustained ventricular tachycardia have been identified as principal predictors of myocardial damage in CCC.62 Additionally, heart rate turbulence, turbulence onset, and turbulence slope are strong risk predictors of sudden death.63 Alternatively, another paper has proposed QT-interval dispersion, syncope, ventricular extrasystoles, and severe dysfunction of the left ventricle as the strongest indicators of sudden death.64
Left atrial volume has been shown to provide powerful prognostic information incrementally and independently of clinical data and conventional ECG parameters, allowing for the prediction of chronic Chagasic disease patient survival.65 Moreover, determination of ACE2 activity provided a new and important diagnostic and prognostic marker for patients with Chagas disease.66
One of the principal problems of the aforementioned prediction factors is their relative complexity for a large-scale survey of Chagasic patients at risk of sudden death/heart failure. The authors have proposed several markers to identify patients at risk of heart failure and/or Chagas disease. Based on the pathophysiological model, the authors have explored serological markers of sudden death and heart failure.
Autoimmunity and Inflammation as Predictive Markers of Sudden Death
Sudden death is the principal cause of death for Chagas disease patients, which is especially concerning because most cases are asymptomatic. A key issue for physicians is identifying and monitoring a marker that can orientate the treatment of asymptomatic patients. Based on recent work, the Cha transcription factor is an interesting candidate for a prognostic marker. Cha is a ubiquitously expressed member of the class C basic helix-loop-helix family; expression of the protein and its binding to the CD2 promoter region negatively correlated with CD2 expression in T cells after mitogenic stimulation, whereas overexpression of Cha inhibited CD2 expression.67 It has been reported that T. cruzi-infected mice contain autoreactive T cells that can cross-react with Cha and the shed acute-phase antigen homologous peptides. Transfer of T cells from infected mice into noninfected counterparts triggered anti-Cha antibody (with an epitope targeting residues 120–129) production in naïve recipients, causing cardiac pathology similar to T. cruzi-infected mice.68 The authors have demonstrated that higher anti-short form Cha antibodies levels are present in Chagasic patients from Colombia and Venezuela with a higher risk of sudden death.69 Measurement of anti-short form Cha antibodies levels, through an conventional ELISA assay, may help to identify non-symptomatic patients at risk of sudden death.
Furthermore, the role of inflammation in arrhythmias was explored using a mathematical model. A multiplex array of cytokines was developed (IL-2, IL-6, IL-4, IL-10, IL-17, TNF, and IFN-γ) to identify discriminant variables to clinical and treatment evolution. IL-2 was identified as the principal discriminatory variable to evaluate sudden death risk and amiodarone treatment response.5 Interestingly, IL-2 release induced stretching of the tissue, which led to the appearance of abnormal bioelectrical activity, causing an increase in action potential duration at the levels of 90% repolarisation.70 Additionally, IL-2 has been associated with an upregulation of SCN3B expression and a resultant increase in sodium current density.71 Myocarditis and near fatal arrhythmias during high dose IL-2 therapy for metastatic renal cancer were recently reported,72 which reinforces the potential use of IL-2 levels as a marker for arrhythmias in Chagasic patients.
Markers of Vascular Function in Chronic Chagasic Cardiomyopathy
CCC evolution must be addressed to identify non-symptomatic patients. A key tool for the assessment of vascular function is L-arginine plasmatic levels. Plasmatic levels of L-arginine and the asymmetric dimethylarginine (ADMA), a competitive inhibitor of NO synthase, have been associated with heart failure, demonstrating that the lower L-arginine to ADMA ratio indicates less available NO and suggesting that NO-related endothelial dysfunction may play a role in the adverse risk of heart failure progression.73 Additionally, the use of the L-arginine to ADMA ratio for evaluating postoperative outcome in patients after heart transplant has been reported.74 For Chagas disease, Carbajosa et al.75 demonstrated reduced plasmatic levels of L-arginine in mice during acute infection and increased survival rates when the mice were supplemented with 3.75 mg/kg of L-arginine in drinking water.75 Based on this previous work, the role of the L-arginine to ADMA ratio as indicator of heart failure in Chagasic patients has been proposed. Some preliminary, unpublished results support this perspective. Based on pathophysiology, Figure 1 and Table 11,4,74-80 summarise the possible serological markers for identification of potential candidates for treatment during Chagas disease.
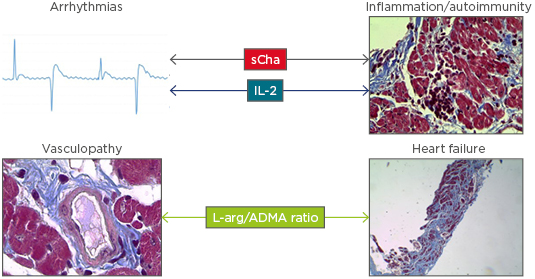
Figure 1: Short form Cha, IL-2, and L-arginine/asymmetric dimethylarginine ratio are proposed as evolution markers for non-symptomatic Chagasic patients.
As described in the main text, sCha (a T cell transcription factor) autoantibodies have been associated with autoreactive T cell infiltration and high sudden death risk in Chagasic patients, being considered a potential prognosis marker for malignant arrhythmias in Chagas disease. On the other hand, IL-2, a potent mitogen of T cells, has been associated with sudden death and atrial fibrillation in Chagasic patients and other cardiac pathologies, suggesting that this cytokine is an inflammatory marker that may be detected during the non-symptomatic phase. These facts are summarised by the arrows connecting markers to the relevant pathophysiological processes. Finally, L-arginine/ADMA ratio is proposed as a marker of endothelial function and may indicate heart failure risk in Chagasic patients.
ADMA: asymmetric dimethylarginine; L-arg: L-arginine; sCha: short form Cha.
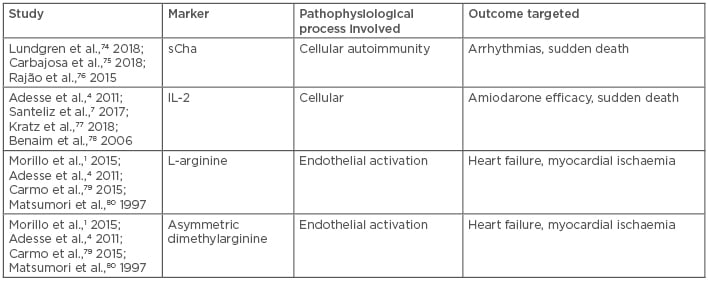
Table 1: Summary of listed evolution markers for chronic Chagas disease.
sCha: short form Cha.
EMPIRICAL RATIONALE FOR AN APPROACH FOR INDETERMINATE AND CHRONIC CHAGASIC CARDIOMYOPATHY PATIENTS
Based on the pathophysiology of Chagas disease and recent work, possible therapeutic alternatives to current treatments that may help to alleviate the effects of Chagas disease are outlined in the following sections.
Benznidazole and Nifurtimox: Old-Fashioned Therapy with a New Approach
BZ and nifurtimox are the standard treatment for Chagas disease. Both therapeutics exploit free-radical generation to generate nitrosative damage in the developing parasite. BZ induces oxidation, mainly in the nucleotide pool, and catalyses the generation of double-stranded breaks in the parasite DNA, caused by the induction of extensive heterochromatin unpacking of the parasite genome. Finally, it has been reported that BZ induces lesions in the mitochondrial DNA.76 As previously stated, one of the most limiting factors preventing BZ use is the high rate of treatment discontinuation due to side effects; furthermore, the therapeutic efficacy of BZ in chronic Chagasic patients is still a matter of debate. As a result, shorter or intermittent dosing regimens of BZ treatment or combining BZ and nifurtimox with new chemical entities have been proposed.77 What follows is a short summary of possible therapeutic alternatives to improve the efficacy of BZ during the treatment of non-symptomatic Chagas disease.
Amiodarone: A Polyvalent Tool for Treatment a Variable Pathology
Amiodarone is considered a ‘dirty’ drug in virtue of the multiple mechanisms of action the drug exhibits. Reported amiodarone actions include a) the inhibition of inward sodium and calcium currents, b) blockage of voltage and ligand-gated potassium channel currents, c) downregulation of Kv1.5 messenger ribonucleic acid (mRNA), and d) nonselective downregulation of β adrenoceptors during chronic administration. Moreover, amiodarone has been postulated as a trypanocidal drug76 with a profound effect on intracellular amastigotes, including mitochondrial swelling and disorganisation of reservosomes and the kinetoplast and a blockade of amastigote-trypomastigote differentiation.4 Statistical differences in parasitaemia were not detected in Chagasic patients treated with amiodarone.80 However, in a large-scale study,1 BZ administration combined with amiodarone appeared to benefit the patient.
Amiodarone is associated with anti-inflammatory effects. Matsumori et al.80 conducted one of the first reports that linked amiodarone and inflammation, showing amiodarone triggered the reduction of TNF release by peripheral blood mononuclear cells.80 This was followed by the proposal that amiodarone dose-dependently exerts a powerful anti-inflammatory activity, possibly due to the activation of NO as a result of calcium channel antagonism, to the inhibition of phospholipase A2, and/or a reduction in neutrophil movement and activation, which is thought to reduce free radical production and proteolytic enzyme release.81 More recently, it was proposed that, through regulation of AP-1 and NFκB signalling, amiodarone inhibits the production of IL-2, TNF, and IFN-γ, and prevents T cell activation.82 This last result is closely associated with the role of IL-2 as an indicator of amiodarone treatment as reported in Chagasic patients,5 and it strongly suggests a possible role of amiodarone treatment beyond channel modulation capacity. The findings make the drug an important tool to consider in the prevention of cardiac remodelling and sudden death because of Chagas disease. The determination of an amiodarone treatment pattern to avoid the chronic toxicity associated with amiodarone treatment is yet to be confirmed.
Angiotensin-Converting Inhibitors: A Cheap Weapon for an Elusive Enemy
The ACE inhibitor (ACEI) family of antihypertensive agents are among those most widely used in antihypertensive therapy. ACEI are particularly effective when reducing proteinuria and improving outcomes in chronic heart failure.83 Beyond their classically reported effects, ACEI have a range of action mechanisms, more of which have been expanded upon in recent years. It has been shown that captopril induces a dose-dependent reduction of total and differential white blood cell counts, while it improved serum oxidant/antioxidant biomarkers and histopathological changes in lipopolysaccharide-treated rats.84 However, there has been doubt cast on the evidence indicating that ACE reduces the plasma level of major inflammatory markers in hypertension models.85
The most promising data have been described for the use of ACEI for Chagas disease. In an experimental approach, it was proposed that a combination treatment of BZ plus enalapril was able to increase the IL-10 levels and reduce the cardiac inflammation while BZ inhibited collagen neogenesis at the infection site86 and reduced cardiac leukocyte recruitment, total collagen in the cardiac tissue, chemokines, creatine kinase, creatine kinase muscle to brain ratio, and C-reactive protein levels in an experimental model of chronic phase disease.87 Conversely enalapril alone treatment alone a reduction in serum levels of IFN-γ, TNF-α, CCL5/ RANTES, and NO, but not in that of IL-10.88 However, in contradiction to other work, Coelho dos Santos et al.89 reported that captopril interferes with the host-parasite equilibrium by enhancing infection of monocytes and decreasing the expression of the modulatory cytokine IL-10, while guiding development of the proinflammatory Th17 subset.
In Chagasic patients, it has been proposed that treatment with enalapril, spironolactone, and the subsequent addition of carvedilol is safe and associated with beneficial effects on cardiac function and clinical status.90 This finding is closely related with results that associate plasma ACE2 activity with their clinical severity and echocardiographic parameters, resulting in a significantly increase in Chagas diseased patients with heart failure but not in patients without systolic dysfunction.66 With these data in mind, it is plausible to propose ACEI treatment, especially in hypertensive patients, in order to control the endothelial dysfunction in indeterminate risk patients and reduce the risk of developing CCC. Figure 2 shows the summary of the treatment proposed to indeterminate Chagas diseased patients.
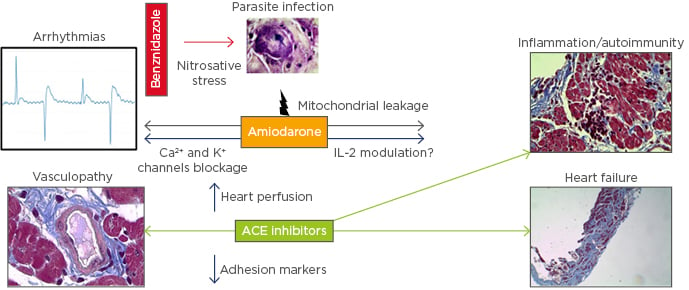
Figure 2: Therapeutic alternative proposed for chronic Chagasic patients.
In addition to parasite replication control with benznidazole, amiodarone treatment may provide a parasiticidal synergistic effect, together with the well-known anti-arrhythmic activity and the recently proposed anti-IL-2 effect. ACE inhibitors have also been associated with a reduction in inflammation, probably due to endothelial transmigration blocking, additional to these effects in preventing cardiac remodelling and heart failure.
ACE: angiotensin converting hormone.
Beta-Blockers and Mineralocorticoids Receptor Antagonists
Beta-blockers and mineralocorticoids are widely used to treat heart failure and cardiac remodelling. These drugs show a wide range of action mechanisms and therapeutic uses and are therefore interesting candidates to be considered for CCC treatment.
Beta-blockers available for clinical treatments have variable affinity for β1 and β2 receptors, and in some cases exclusively for β3, although there is an overall emphasis on the β1 receptor, the most prevalent subtype of adrenergic receptors in the heart. Carvedilol is a nonselective beta-blocker indicated in the treatment of mild-to-moderate congestive heart failure. It blocks β1 and β2-adrenergic receptors as well as the α1-adrenergic receptors. In experimental models, carvedilol did not attenuate cardiac remodelling or mortality in this model of Chagas cardiomyopathy, but the treatment did improve survival during the acute phase of the disease.91 Moreover, in a c57bl/6 mouse model of infection, carvedilol therapy did not alter the levels of circulating parasites. Instead, the drug modulated the pattern of CCL2 and IL-10 mediators.92 An extensive meta-analysis, however, concluded that there were no conclusive data to support or reject the use of either carvedilol for treating Chagas cardiomyopathy,93 although individual clinical trials suggest beneficial effects.94,95 Interestingly, carvedilol alone and in combination with vitamins C and E was effective at attenuating the systemic oxidative stress in patients with Chagas heart disease, especially those less severely affected, thus suggesting the possibility of synergism between these compounds96 and opening a window for future explorations.
Propranolol is a widely used noncardioselective β-adrenergic receptor antagonist used in the treatment and prevention of many disorders, including acute myocardial infarction, arrhythmias, angina pectoris, hypertension, hypertensive emergencies, hyperthyroidism, migraine, pheochromocytoma, menopause, and anxiety. Recently, propranolol has been linked to the impairment of lysosome spreading and prevention of T. cruzi invasion in HeLa cells.97 However, despite this evidence, the development of a sufficiently extensive clinical programme that allows for the establishment of beta-blockers’ clinical efficacy in treating CCC remains elusive.
Finally, mineralocorticoid receptor antagonists (MRA) are often used as potassium preserving diuretics in the treatment of heart failure and ascites. However, there are few reports about the use of MRA for treating CCC. In experimental models, it has been proposed that spironolactone attenuated myocardial remodelling in Chagas cardiomyopathy, reduced mortality during the chronic phase, and reduced inflammatory infiltration in a Sirius hamster model.98 Interestingly, a report that suggested the improvement of cardiac function in CCC patients cotreated with spironolactone90 and ACE inhibitors was highlighted, suggesting MRA as adjuvant therapy in CCC heart failure. It is necessary to generate more evidence to determine the efficacy of MRA in treatment of CCC.
CONCLUSION
Treatment of indeterminate patients is one of the most complex issues in Chagas disease and represents a challenge for clinicians. This review explored an approach for treating Chagasic patients during indeterminate phase considering several variables: a) pathophysiology of CCC, b) experimental and clinical reports, and c) accessibility of treatment for CCC. It is especially important to consider the fact that, in the longest clinical trial to date (BENEFIT),1 BZ treatment alone was not able to improve clinical outcome, supporting the proposal of combination parasiticide therapy with drugs that allow for the improvement of vascular function, the modulation of the immune response, electrophysiological alteration in CCC, and, importantly, that are associated with putative serological markers for evaluating drug efficacy. In this review, alternative therapeutics (amiodarone and ACEI) that may be considered as first-line treatment when the clinical indication for their original usage exists were highlighted. Amiodarone, in particular, is a plausible option for patients with reduced ejection fraction and/or symptomatic arrhythmias, when clinically indicated,99 and, when feasible, therapy should be combined with ACEI agents to reduce cardiac remodelling through immunomodulation or inclusive parasite replication control. The currently available data may allow for the introduction of these new approaches without expensive trials. However, it may be necessary to conduct more extensive research in order to define treatment strategies, possible adverse effects, and, if it is necessary, to develop different therapeutic schemes for the mixed clinical presentations (gastrointestinal or cardiac forms) or for patients with concomitant cardiovascular pathologies (diabetes, atherosclerosis, or hypertension).








