Abstract
Considered initially as a bystander, tricuspid regurgitation has shown to be an important predictor of mortality in patients with left-side valvular or myocardial disease. However, a sizeable number of patients remain untreated until the end stage when cardiac surgery presents a prohibitive risk. The emergent need in finding a treatment for patients with tricuspid regurgitation deemed for surgery options have encouraged the development of transcatheter tricuspid valve interventions. These procedures mimic classical surgery techniques and they are mainly divided in two categories: repair (annuloplasty, coaptation devices, edge-to-edge techniques) and transcatheter tricuspid valve replacement. This review aims to provide an updated overview and a clinical perspective on novel transcatheter tricuspid valve interventions, highlighting potential challenges and future directions.
INTRODUCTION
In the last two decades, the interest in tricuspid valve (TV) treatment has increased;1,2 nevertheless, there is still a large percentage of the population with tricuspid regurgitation (TR) who do not receive a surgical treatment because of the high-risk profile. Patients with untreated TR have a poor prognosis3 and most of them receive lifetime medical therapy until intractable right heart failure and end-organ dysfunction appear.
Valve regurgitation remains the principal pathology of the TV and it is more often secondary rather than caused by a primary valve lesion. Annular dilatation and increased tricuspid leaflet tethering in relation to high right ventricular (RV) pressure and/or volume overload cause secondary TR. Left-sided heart disease, atrial fibrillation, or pulmonary hypertension are frequently involved in the pathogenesis of TR.4 All these evidences changed the management of TR into a more aggressive surgical approach, and the most recent guidelines recommend surgical repair of concomitant replacement during left valve surgery, even in patients with tricuspid annular dilatation or recent signs of right heart failure with non-severe TR.5
Despite the improvement in operative techniques, the in-hospital mortality in patients with combined surgery or isolated TR who underwent surgical replacement (12.6% and 7.1%, respectively) or repair (10.8% and 8.1%, respectively) is still high.6 Moreover, previous TV surgery recurrence of moderate or severe TR may be as high as 60% at 5 years7 and reoperation is necessary in approximately 20% of patients within 10% after TV surgery.8 While redo surgery is the treatment of choice for a degenerated bio-prosthesis or deterioration of the ring annuloplasty, it may be associated with a very high mortality rate reaching 35% at 30 days,9 particularly in patients with comorbidities.
Patients with TR and high risk for surgery (multiple comorbidities, advanced age, RV dysfunction, previous surgery) were until recently predestined to conservative treatment. The promising results in the field of aortic and mitral valve percutaneous interventions in high-risk patients have encouraged the development of percutaneous tricuspid interventions. Nevertheless, percutaneous treatment of TR is a more complex procedure, and therefore better understanding of TR mechanism is fundamental.
Shortly, the pathophysiology of functional TR can be divided into three phases. Initially, left-side heart disease, may determine impairment of RV, with progressive dilatation, which can lead to tricuspid annulus (TA) enlargement. In the second phase, the progressive dilation of the RV and TA can result in a poor leaflet coaptation leading to significant TR. Finally, in the third phase continuous distortion of RV geometry especially on the anterior wall associated with tethering of the leaflets will get worse the degree of TR.
Unfortunately, TR has a silent evolution and patients are often referred in the latest phase, when they present with RV dysfunction and an important gap coaptation.
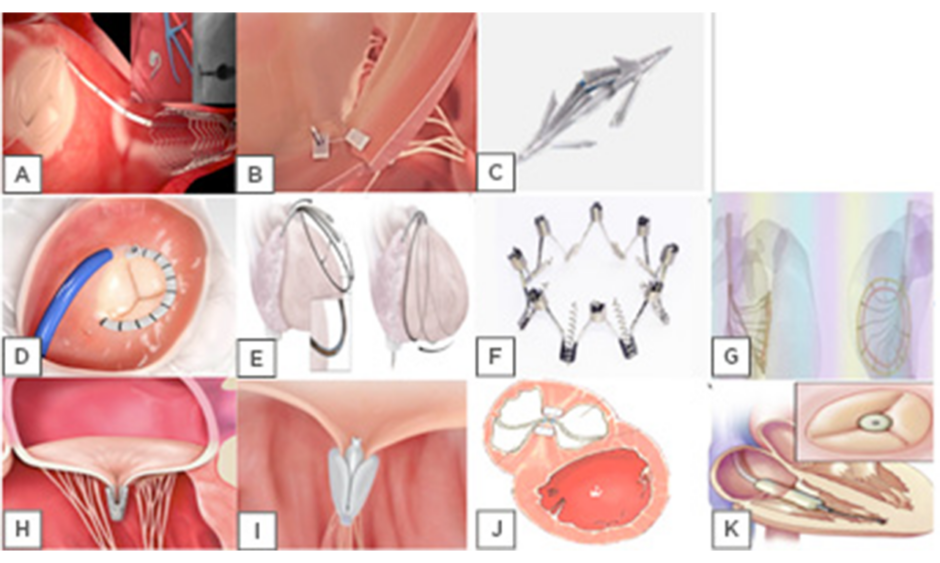
Until now, >18 devices have been developed or are under development for the pathologic tricuspid apparatus treatment (Figures 1 and 2). Based on type of procedure (repair or replacement) they are divided in four categories: TV annuloplasty devices (suture-based or rings), coaptation devices, edge-to-edge techniques (Figure 1), and transcatheter TV replacement (orthotopic and heterotopic-caval valve implantation) (Figure 2).
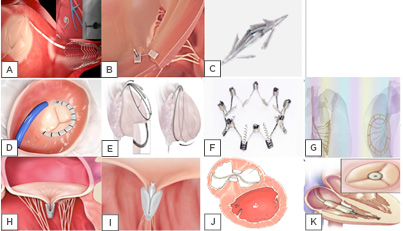
Figure 1: Tricuspid valve repair. Tricuspid annuloplasty devices: ‘Suture based’; A) Tricinch; B) Trialign; C) MIA. ‘Rings based’; D) Cardioband; E) Traipta; F) Milipede; G) Da Vingi. Edge-to-edge techniques devices;H) Mitraclip; I) PASCAL;J) PASTA. Coaptation devices; K) Forma.
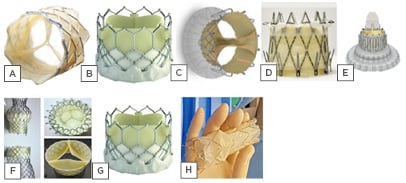
Figure 2: Devices for percutaneous tricuspid valve replacement. Orthotopic valve implantation: A) Melody; B) Sapien; C) Trisol; D) Navigate; E) Lux-Valve. Heterotopic valve implantation; F) TricValve; G) Edwards Sapien; H) Tricento.
Few of these were previously successfully used in percutaneous mitral valve interventions and they were transferred to TV.10,11 Nevertheless, the majority of the studies performed with these devices are in the initial phase (first in humans and safety and feasibility studies).
TRICUSID VALVE ANNULOPLASTY DEVICES
The TriCinch System Device
The 4Tech TriCinch™ Coil System (4Tech Cardio Ltd., Galway, Ireland) is a novel percutaneous device for severe functional TR designed to reduce TA dimensions. An anchor is placed in the anteroposterior annulus and connected to a stent, which is then implanted into the inferior vena cava (IVC). The applied traction force between both reduces the annulus dimension and TR. In the PREVENT trial (early feasibility study, 24 patients), the anchoring system (stainless steel corkscrew) was implanted in the anteroposterior portion of the TA. The procedural success was 75%. At the 6-month follow-up, 75% of patients had functional Class I or II.12
The clinical trial ‘Evaluation of the Percutaneous 4Tech TriCinch Coil Tricuspid Valve Repair System’13 will include 90 patients with significant functional TR and high risk for surgery. The main objective is to prove safety and performance of the newest Tricinch Coil System device (the stainless steel corkscrew was replaced by coil system which is implanted in the anteroposterior commissure and externalised in the pericardial space).
Trialign Device
The Trialign system (Mitralign Inc., Tewksbury, Massachusetts, USA) attempts to replicate the results of the current modified Kay annuloplasty (conversion of an incompetent TV into a competent bicuspid valve). During the procedure, two polyester pledgets are anchored at the TV annulus in the posteroanterior and posteroseptal positions and cinched together to obliterate the posterior leaflet.14 Initially, only one pair of pledgets was implanted for each patient but later, in patients with very large annulus, multiple pledgets were used (‘side by side’ or ‘in series’). The USA early feasibility study SCOUT I10 included 15 patients with 93% procedural success and 30-day technical success of 80%. A significant reduction of the TA diameter and the regurgitant orifice area as well as an improvement in the patients’ symptoms was observed at the 30-day follow-up. Currently, SCOUT II CE Mark study is enrolling 60 patients in different centres in Europe and the USA with favourable preliminary results.15
Minimally Invasive Annuloplasty Device
Minimally invasive annuloplasty (MIATM) is a transcatheter tricuspid annuloplasty device designed to reproduce, percutaneously, the open surgical bicuspidisation procedure of the TV. It is composed of a thermoplastic elastomer (MyoLast) and low mass polymeric, compliant, self-tensioning anchors (PoliCor).
The catheter-based system provides a customisable number of implants deployed to the target annulus, allowing further catheterisation, or surgery if it is needed. The device is surgically implanted through a 16 F steerable delivery system. The first of 40 patients in the STTAR study were already successfully treated.4
Cardioband Device
The Cardioband repair system (Edwards Lifesciences, Irvine, California, USA) was initially designed for the treatment of secondary mitral regurgitation (MR); the results showed a 95% MR reduction (MR ≤2) sustained at 1-year follow-up.16 Moreover, the device was successfully used in patients with severe functional TR, receiving the CE Mark approval in May 2018 (the only transcatheter device for tricuspid regurgitation with CE Mark approval). The results from the TRI-REPAIR17 study were recently presented, showing echocardiographic and clinical parameter improvements at 6 months follow-up. The device is designed as a percutaneous annuloplasty band using a transfemoral approach. Cardioband implantation starts next to the anteroseptal commissure and continues along the anterior leaflet until anteroposterior commissure. After Cardioband cinching, the device reduces the TA dimensions.
Transatrial Intrapericardial Tricuspid Annuloplasty Device
The action mechanism of Traipta (transatrial intrapericardial tricuspid annuloplasty) device is based on an extracardiac tricuspid annuloplasty. The device is positioned in the pericardial space and delivered by puncture through the right atrial appendage.18 Pericardial access is obtained by puncturing the RA appendage from within, after transfemoral venous access. An adjustable circumferential implant, which exerts compressive force over the annulus, is delivered along the AV groove within the pericardial space. Tension on the implant is then adjusted interactively to modify TA geometry and thereby reduce TR. The RA puncture is then sealed using a nitinol closure device.
Preclinical experience in animals showed good safety of the implant with significant annular area reduction.18 There are still issues to address such as coronary artery compression and control of the pericardial sheath before first-in-human testing can occur.
The MillipedeTM System
The Millipede system (Millipede, LLC, Ann Arbor, Michigan, USA) is a repositionable and retrievable complete ring, which can be implanted surgically or via a transcatheter on the atrial side of the native TA to restore its shape and diameter. Designed initially for the mitral valve, it was used successfully in two cases for TR with significant reduction of annulus dimension (36%) and TR grade.19
DaVingiTM Tricuspid Regurgitation System
This is a two-step procedure using a novel annuloplasty approach based on the tissue-healing process to achieve a strong neoannulus and to allow an aggressive annular reduction. During the first procedure, a direct percutaneous annuloplasty is performed. Predictable annular physiologic constriction using an adjustment tool takes place in a second stage (90 days) after a period of tissue healing. To date, four cases have been performed in a first-in-human study.20
COAPTATION DEVICES
FormaTM Device
The Forma Repair System (Edwards Lifescience) is a valve spacer, which is positioned into the regurgitant orifice to create a platform for native leaflet coaptation. The device is delivered through axillary venous access and is then distally anchored to the RV apex. It is a fully retrievable device until sheath removal. Proximal fixation is obtained in a small surgically prepared pocket. The results of 1-year follow-up of SPACER-trial in 18 patients showed a reduction of TR and improvement in New York Heart Association (NYHA) functional class and 6-minute walk test. One patient presented device thrombosis at 4-month follow-up.21
MitraClipTM Device
More than 650 procedures have been performed worldwide, and the multicentre TriValve registry showed that this therapy is so far the most frequent technique applied for percutaneous TR treatment.22 Preliminary evidence suggests that MitraClip is safe, feasible, and associated with an improvement in NYHA functional class and 6-minute walking distance at short-term follow-up. In a recent study, the procedural rate success was 81%.22 Small TR coaptation gap size and central/anteroseptal TR jet locations were identified as independent predictors of procedural success and coaptation gap >10 mm, ORE >0.6 cm2, tenting area >2.1 cm2, and TV vena contracta >11 mm as predictors of unfavourable TR repair.22 In patients with severe mitral and tricuspid regurgitation, concomitant MitraClip appears to improve functional status and biventricular haemodynamics early after the intervention and mid-term follow-up.11 Moreover, TRILUMINATE CE Mark trial23 enrolled 85 patients over 25 centres in Europe, Canada, and the USA, and preliminary results will be available soon.
PASCALTM Device
The Edwards PASCAL transcatheter mitral valve repair system (Edwards Lifesciences) integrates technical aspects from the Forma and the MitraClip devices by combining a 10 mm central spacer and two paddles (25 mm width) and clasps (10 mm length) that attach the device to the valve leaflets, thus overcoming possible limitations of the former devices separately. In patients with severe MR, it showed to be a feasible option in preliminary efficacy data.24 The first successful case treating severe TR with PASCAL device was recently reported.25
Pledget-Assisted Suture Tricuspid Annuloplasty Device
The pledget-assisted suture tricuspid annuloplasty (PASTA) device reduces the TV orifice by opposing septal and lateral targets on the TA using percutaneously pledged sutures. The specific annular targets for PASTA device are the mid-anterior leaflet and the posterioseptal leaflet commissure. Because there is no anatomic septal annulus, the septal pledget target incorporates interventricular septal myocardium between the base of the septal leaflet and the coronary sinus. The result is a double-orifice TV. Preliminary studies in animals showed a reduction in annular area and TR; nevertheless, serious complications were common in this technical development study but were mostly related to apical access.26
TRANSCATHETER TRICUSPID VALVE REPLACEMENT (ORTHOTOPIC CONCEPT)
Melody and Edwards SapienTM Tricuspid Valve for Valve-in-Valve and Valve-in-Ring
Patients with previous TV repair or replacement who require a tricuspid reintervention have a prohibitive surgical risk.9
Transcatheter valve implantation has been successfully implanted via the transatrial, transjugular, or transfemoral approaches. Two different transcatheter heart valves have been successfully implanted during tricuspid valve-in-valve or valve-in-ring procedures: the Edwards Sapien valve and the Melody valve (Figure 2). The biggest registry of transcatheter TV replacement included 306 patients (284 patients with valve-in-valve and 22 patients with valve-in-ring).27 Post-procedure, 83% of the patients presented none or trivial TR. During follow-up (3 years), 31 patients (10%) underwent re-intervention on the TV and survival rate was 83%.
NAVIGATE, TRISOL, LUX-VALVE IN TRICUSPID
NaviGateTM
The NaviGate bioprosthesis is a novel self-expanding valved stent designed to treat functional TR. The preclinical evaluation showed that the NaviGate device is safe, feasible through two different approaches with a stable engagement of the native annulus, and has excellent haemodynamic and valve performance. The configuration of the stent is specifically designed in a geometry that engages the TA and TV leaflets from both inferior and superior aspects and maintains a minimal extension into both the atrium and ventricle to avoid flow dynamics alterations. Notably, 27 patients received NaviGate valve with excellent results and low rate of paravalvular leakage; nevertheless, 30-day mortality was 11%. Although the preliminary results are promising, there are some pitfalls that still have to be addressed, such as device anchoring and sealing (large asymmetric annulus, minimal calcium), delivery system size (42 OD), and leaflet durability (risk of thrombosis).28
Trisol
Trisol valve is a percutaneous valve design for TR taking into consideration the RV afterload after the valve implantation. The valve apparatus is built as a single bovine pericardial piece, attached to the nitinol frame in two opposite central commissures, and functions as two separate leaflets. The leaflets move to the centre of the lumen during diastole, enabling two large lumens for the diastolic filling of the RV. During the systole the pericardium (one big leaflet) acquires the dome shape, which increases the closing RV volume with pressure relief and function preservation. Further investigations are necessary before the first-in-human studies are performed.29
LUX-ValveTM
The LUX-Valve (Jenscare Biotechnology, Ningbo, China) is a self-expanding bovine pericardial tissue valve mounted on a nitinol stent frame with transatrial access. The device has a self-adaptive skirt to minimise paravalvular leakage and a mechanism for secure anchoring within the RV. To date, only experimental data are available.4
CAVAL VALVE IMPLANTATION (HETEROTOPIC CONCEPT)
In patients with limited tricuspid therapeutic options, an alternative approach to percutaneous treatment of TV is to implant transcatheter prosthesis in IVC (single valve approach) or in combination with a superior vena cava valve (dual valve approach) to prevent caval backflow of TR and mitigate systemic venous congestion. In the presence of advanced RV dysfunction, the single valve approach appears to be safer compared with the dual-valve approach because it reduces RV preload, but single valve implantation is potentially less effective regarding haemodynamic and clinical improvement because of collateral circulation. More than 40 patients benefited from Caval Valve Implantation (CAVI) prosthesis using Edwards Sapien valve or Tricvalve, of whom the majority were compassionate cases.30 Published data,30 in an early clinical experience, showed improvement in NYHA class and haemodynamic parameters but 1-year mortality was high (63%).
The main advantage of this procedure is that it is easy to implant through transfemoral access.The safety and efficacy of Edwards Sapien valve implantation at the IVC is currently being studied in the TRICAVAL31 and HOVER32 trials (Figure 2).
TricentoTM Device
The Tricento transcatheter heart valve is composed of a bicavally-anchored covered stent with lateral bicuspid valve element (to the right atrium) made of thin porcine pericardium leaflets requiring only a low closing pressure. The device, as with other heterotopic devices, aims to abolish the systolic backflow in both the inferior and superior caval veins.Because there is a great amount of variability regarding the anatomy of the caval veins and the right atrium, the stent needs to be custom made. The first-in-human data was recently published showing reduction of caval vein regurgitant volume with stable position in the follow-up (Figure 2).33
CLINICAL PERSPECTIVES ON TRANSCATHETER TRICUSPID INTERVENTIONS
There is no doubt of the necessity to find treatment options for severe TR. In the last decade, the number of devices destined to treat severe TR multiplied. The TV is no longer the ‘forgotten valve’ and today’s clinicians are witnesses of a real device parade (Figure 1). Some of the devices were successfully used in mitral or aortic valve percutaneous treatment (MitraClip, Cardioband, Mitralign, PASCAL, Millipede devices, Edwards Sapien, and Melody valve). Moreover, a big proportion of devices are still in the early development stages and long-term follow-up data are not available. The only device with a CE Mark for TR is Cardioband; others such as Tricinch, Trialign, MitraClip, or Forma are still enrolling patients in CE Mark trials.
Most percutaneous annuloplasty devices reproduce well-established surgical techniques and they are divided into suture-based and rings. The acute results are encouraging, showing reductions in annulus dimension and improvements in quality of life and symptoms (Table 1).
For those cases with massive TR with a big gap between the leaflets, the Forma device also showed improvement in quality of life parameters and symptomatology.
Edge-to-edge techniques, particularly MitraClip, have become the first-choice approach for high-risk patients with functional TR, likely because of wide availability and operator familiarity.The other two devices (PASCAL and PASTA) are still in the early stages of development.
For situations in which tricuspid repair is not possible, five valves were designed for percutaneous tricuspid replacement (two of them are also available for valve-in-valve and valve-in-ring procedures) (Figure 2). Special precautions are taken into consideration during valve implantation and different strategies were proposed to avoid A-V node conduction system damage. The Trisol valve brings a new concept regarding the RV closing volume, which permits pressure relief and function preservation of RV. Despite the complex anatomy of the TA, only a small percentage of cases presented residual TR after valve implantation (Table 1).
The three heterotopic valves available are designed to relieve the symptoms and reduce the backflow in the caval veins. Nevertheless, this therapy was used in pluripathologic patients, the majority of whom were compassionate cases and the mortality rate was >50% at 1-year follow-up because of patients’ pre-existing conditions.
All these therapies showed promising results in terms of acute procedural success but follow-up data are missing in most of them. Despite the acute procedural success rate, technical details such as sheath size (Table 1), possible anatomic complications, and the pre-existing leads in RV should be taken into consideration.
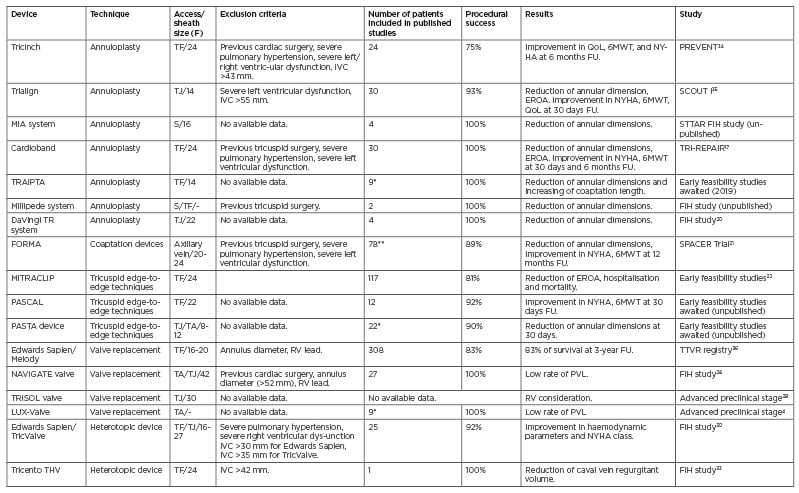
Table 1: Summary of transcatheter tricuspid valve devices and their general characteristics and trial results.
6MWT: 6-minute walk test; F: French; FIH: first in human; FU: follow-up; IVC: inferior vena cava; NYHA: New York Heart Association; RV: right ventricle; PVL: paravalvular leakage; S: surgical; TA: transatrial; TF: transfemoral; TJ: transjugular. *Studies in animals **Results available in 18 patients
There is still a paradox between the TR prevalence, surgical, and percutaneous treatment.Only a few patients with severe TR, high risk, and who are deemed for surgery are suitable for first-in-human or early feasibility studies. The majority of the enrolling studies are excluding real symptomatic patients with pulmonary hypertension, severe RV dysfunction, or severe left ventricle dysfunction.
CONCLUSION
TV is no longer the ‘forgotten valve’: for patients with severe TR and high surgical risk, several percutaneous options are available. Nevertheless, all these therapies are in a growing phase and not all possible candidates are suitable for these new techniques.