Meeting Summary
Despite the use of guideline-directed medical therapy (GDMT) for heart failure (HF) with reduced ejection fraction (HFrEF), significant unmet need exists in patients with worsening HF who remain at high-risk of recurrent events. During these two symposia, leading cardiology experts explored therapeutic advances for patients with symptomatic chronic HF following a worsening HF event, focusing on the new soluble guanylate cyclase stimulator vericiguat. Key outcomes data from the VICTORIA trial show that vericiguat affords significant clinical benefits in this patient population and has an important role to play in the future treatment landscape for worsening HF.
SYMPOSIUM 1
New Therapies for Patients Following a Worsening Heart Failure Event
Patients with HF embark on a vicious cycle characterised by progressive worsening over time, recurrent hospitalisations associated with deteriorating cardiac function, and eventual death.1,2 This worsening HF is a component now recognised as increasingly prevalent, noted Armstrong, but has “not yet achieved the clinical attention it deserves.” Despite the use of best evidence-based GDMT, patients with HFrEF continue to remain at significant residual risk of both cardiovascular (CV) death and HF hospitalisation (HFH).3-7
Unlike existing therapies that target established activated disease pathways in HF, vericiguat represents a new therapeutic approach that stimulates soluble guanylate cyclase (sGC) to restore the impaired nitric oxide (NO)-sGC-cyclic guanosine monophosphate (cGMP) pathway (Figure 1).1,8-14 Armstrong explained that it is the “conspiracy” between endothelial dysfunction and oxidative stress that leads to intracellular NO deficiency, thereby impairing the cell’s inability to generate cGMP and protein kinase G (PKG). Vericiguat is a direct sGC stimulator and also recruits residual NO in the cell, thereby accentuating this novel pathway and restoring the heart and the vasculature to a more normal state.8,15-18
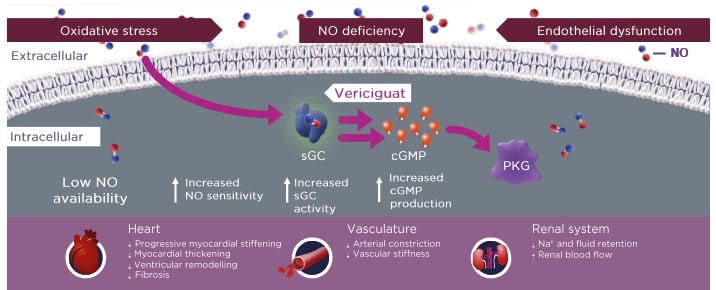
Figure 1: Vericiguat increases soluble guanylate cyclase activity to improve myocardial and vascular function.1,8,12-14
cGMP: cyclic guanosine monophosphate; Na+: sodium ion; NO: nitric oxide; PKG: protein kinase G; sGC: soluble guanylate cyclase.
Therapeutic Advances: A New sGC Stimulator for Patients Following a Worsening Heart Failure Event
Pieske introduced the VICTORIA study, a state-of-the-art, international, randomised, parallel-group, placebo-controlled, double-blind, event-driven Phase III trial, whose objective was to evaluate the effect of vericiguat in patients with symptomatic chronic HF following a worsening HF event.16,19 Eligibility criteria included HFrEF with a left ventricular ejection fraction (LVEF) <45%, New York Heart Association (NYHA) Class II–IV, and elevated natriuretic peptides (stratified by the presence of atrial fibrillation [AF] or sinus rhythm). Renal dysfunction was permitted down to a ”very low” estimated glomerular filtration rate (eGFR) cut-off of 15 mL/min/1.73m2, remarked Pieske. In order to qualify for the VICTORIA study, patients were required to have experienced HFH within 6 months or undergone intravenous (IV) diuretic treatment for HF within 3 months. In total, 5,050 patients were randomised 1:1 to vericiguat or placebo, and uptitrated to a target dose of 10 mg once daily (achieved in approximately 90% of patients in both arms after 12 months). The primary endpoint was time to first occurrence of the composite of CV death and HFH.16,19
Pieske described key baseline characteristics of VICTORIA patients: mean age was 67 years, approximately one-quarter were female, and two-thirds were white.19 Importantly, two-thirds of participants (66.9%) had been hospitalised for decompensated HF within the past 3 months, 17.2% within the past 3–6 months, and 15.9% had received IV diuretics for a worsening HF event within the prior 3 months. Around 40% of patients had an NYHA Class III or IV at baseline, illustrating that patients were more advanced in their HF disease journey, added Pieske. Overall, 10% of the total patient population had eGFR <30 mL/min/1.73m2 and median N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) was 2,816 pg/mL. Around 60% were already receiving triple therapy for HF.19
In the VICTORIA trial, vericiguat significantly reduced the annualised absolute rate of time to HFH or CV death, meeting the study’s primary endpoint (Figure 2).19 The annual event rate was 37.8 events per 100 patient-years in the placebo arm. Pieske described this as “very high,” with over 35% of patients experiencing a primary event of CV death or first HFH after 1 year. In contrast, patients treated with vericiguat achieved a significant reduction in the primary endpoint (hazard ratio [HR]: 0.90; 95% confidence interval [CI]: 0.82–0.98; p=0.02), translating to an absolute rate reduction (ARR) of 4.2 events per 100 patient-years and a number needed to treat (NNT) of 24.19 These data are comparable to ARR outcomes from other recent HFrEF trials, remarked Pieske.
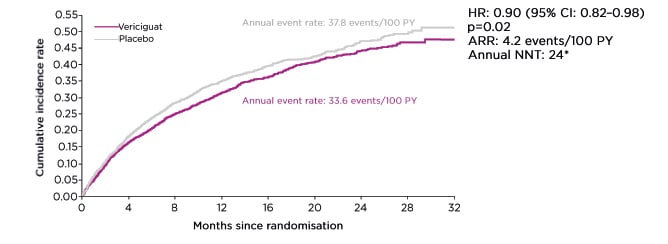
Figure 2: Primary efficacy endpoint from the VICTORIA trial.19
Time to CV death or first HFH. Median treatment duration for primary endpoint: 10.8 months.
CI: confidence interval; CV: cardiovascular; HFH: heart failure hospitalisation; HR: hazard ratio.
Secondary outcomes were in line with the primary endpoint, with vericiguat achieving a significant reduction in total HFH (p=0.02; HR: 0.91; 95% CI: 0.84–0.99) and the composite of first HFH or all-cause mortality (p=0.02; HR: 0.90; 95% CI: 0.83–0.98) compared to placebo.19 Analysis of primary composite endpoint outcomes showed a consistent benefit of vericiguat across a range of prespecified patient subgroups, with no impact of pretreatment with sacubitril/valsartan or baseline eGFR on vericiguat efficacy. There was, however, a significant interaction of NT-pro-BNP levels at baseline, with patients in the lowest three quartiles deriving most benefit from vericiguat treatment.19
Pieske described the safety of vericiguat in the VICTORIA trial as “excellent,” with both the overall adverse event (AE) profile and the incidence of serious AEs proving very similar to placebo. In terms of key AEs of interest, symptomatic hypotension (9% versus 8%) and syncope rates (4% versus 3%) were slightly higher with vericiguat versus placebo; however, no significant differences were noted between the study arms.19
Top Tips for Practical Patient Management
Top tips for the practical management of worsening HF with vericiguat were provided by Senni, who focused on three key phenotypes that commonly present in clinical practice: patients at risk of hypotension, patients with renal impairment, and patients with hyperkalaemia.
Looking at hypotension, data from the VICTORIA trial showed only very small differences in mean systolic blood pressure (SBP) values between patients treated with vericiguat and placebo.19 These decreases in SBP occurred very early in the titration phase and then remained stable throughout the rest of the study, with no further clinically relevant reductions in BP observed.19 No excessive BP reductions were seen with vericiguat in patient groups in VICTORIA at elevated risk of hypotension such as elderly patients (>75 years of age), those with SBP <110 mmHg, or those taking sacubitril/valsartan, noted Senni.20 Moreover, the benefit of vericiguat versus placebo on the primary endpoint was similar across the spectrum of baseline SBP.20
Considering the issue of renal impairment, the impact of vericiguat on renal function trajectories in VICTORIA was found to be similar to that of placebo based on changes in both eGFR and creatinine levels over time.21 Vericiguat also provided a benefit in clinical outcomes of HFH or CV death, HFH or all-cause death, and CV death in patients across all eGFR categories (≤30, >30–≤60, and >60 mL/min/1.73m2).21
For the third and final phenotype, the incidence of hyperkalaemia in VICTORIA was found to be similar between treatment arms, even in patients with low renal function (eGFR ≤30 mL/min/1.73m2), where 8.0% of vericiguat-treated patients experienced hyperkalaemia compared to 10.2% on placebo. Compared to placebo, vericiguat also showed no impact on sodium or potassium levels over time.21
Overall, these data from VICTORIA indicate that vericiguat can be used in clinical practice for the management of worsening HF in patients at risk of hypotension, patients with renal impairment, and patients with hyperkalaemia, Senni concluded.
Questions and Answers
Lam opened the panel discussion segment of the symposium by highlighting important recent changes to European Society of Cardiology (ESC) guidelines, which now recognise worsening HF for the first time and recommend a sGC stimulator as a new treatment option on top of GDMT.22 Vericiguat received a Class IIb recommendation from the ESC and may be considered for patients in NYHA Class II–IV who have had worsening HF, despite treatment with an angiotensin-converting enzyme inhibitor (or angiotensin receptor-neprilysin inhibitor), a β-blocker, and a mineralocorticoid receptor antagonist to reduce the risk of CV mortality or HFH.22 This marks a “huge milestone,” said Lam, with the addition of vericiguat providing an important opportunity to optimise therapy for patients with worsening HF.
Against the backdrop of new ESC guidelines, Pieske outlined how he would manage a patient with chronic worsening HF. The guidelines now acknowledge the entity of worsening HF, where HFrEF patients experience recurrent events despite good background therapy. For these patients, clinicians are “mandated” to provide optimal therapy with the addition of vericiguat, Pieske pointed out. The safety profile is favourable and vericiguat is now approved by both the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) regulatory bodies, so cardiologists will soon have it “at hand” for this important, high-risk patient population, he added.
In the setting of the HF outpatient clinic, Senni was asked where he saw the window of opportunity for vericiguat and any particular circumstances that might dictate prescribing. He outlined practical considerations for starting vericiguat after HFH, which include the need for stable background medical therapy, BP >100 mmHg, and a stable condition in terms of congestive signs and symptoms (making it essential to evaluate patients’ fluid status first). Treatment should be uptitrated from 2.5 mg every 2 weeks to reach the target maintenance dose of 10 mg/day. In the event of development of hypotension, concomitant medications such as calcium blockers, nitrates, or α-blockers may be stopped, and consideration given to reducing diuretic therapy. These steps can allow you to move forward with a successful uptitration of vericiguat, explained Senni. On the issue of comorbidities, data show that the clinical benefits of vericiguat endure irrespective of AF or anaemia status, worsening renal function, or hypotension risk.
Armstrong gave his perspectives on how the VICTORIA study has helped to advance the field of HFrEF management. He described the entity of worsening HF as “extraordinarily important, increasingly common, and imposing a high burden of morbidity and mortality.” Yet it has not been well studied previously in dedicated clinical trials. The discovery of vericiguat has opened up NO-sGC-cGMP as a new therapeutic pathway, explained Armstrong, and by targeting that pathway we have seen a “remarkable,” absolute reduction in CV death and HFH events comparable to the well-known foundational therapies. Vericiguat is an easy-to-use, well tolerated, once-daily medication that can be used without laboratory monitoring for renal function or hyperkalaemia. It represents ”a new arrow in our quiver,” said Armstrong, and an important option for patients that are either unable to tolerate standard-of-care therapy or have broken through with new symptoms.
Lam drew the first symposium to a close by reiterating the urgent, unmet need that exists in patients with worsening chronic HFrEF, who have failed GDMT and require intensification of their HF treatment. Now that the regulatory approvals for vericiguat have been granted and ESC guidelines recommendations received, it is time to start thinking about implementation in clinical practice, she concluded.
SYMPOSIUM 2
What Is Next for Your Patients Following a Worsening Heart Failure Event?
This follow-up symposium provided a practical perspective on identifying patients with worsening HF in the clinic and intervening with vericiguat to optimise therapy and improve outcomes.
When managing worsening HF, it is important to recognise what part of the HF trajectory patients are on, explained Lam. The phase of worsening HF despite optimal medical and device therapy is where “we can really make a difference,” she stressed, before patients progress to advanced HF risk, which is refractory to GDMT. Worsening HF events are characterised by progressive signs and symptoms of HF, for which medical treatment is warranted despite the use of GDMT. Events can manifest as either HFH, the requirement for IV diuretics (regardless of setting), or the need for an urgent outpatient HF visit.23-25 These events are very high-risk and characterised by a progressive reduction in median survival with each hospitalisation.26 Increased mortality risk is evident regardless of care location, seen equally in worsening HF events that occur outside the hospital setting.23
Worsening HF is both a common and prognostically important condition, continued Lam. The PINNACLE Registry® of over 11,000 adults with newly diagnosed symptomatic chronic HF found that 17% developed symptomatic chronic HF following a worsening HF event.24 Hospitalisations were also shown to accumulate for patients with worsening HF. Overall, 56% of patients were re-hospitalised within 30 days of their worsening HF event and the number of HFHs increased with time.24 One in five patients died within 2 years of the event.24
Residual risk, therefore, remains in patients with HFrEF despite the use of existing medications, underscoring the need for new therapies. If patients are manifesting worsening HF “we really need to act,” insisted Lam. “It’s not just a matter of optimising diuretics, it’s about changing patients’ foundational therapies by adding new therapies that work.”
VICTORIA in Context: Deep Dive into the Latest Data
Taking a deep dive into the VICTORIA trial, Butler contextualised the clinical outcomes with a focus on the impact of key patient comorbidities such as chronic kidney disease. He explained that the VICTORIA trial exclusively targeted a population of patients with worsening HF, thereby differentiating it from other trials in the HFrEF space.19 Other elements of the trial design were also different. While most other HFrEF studies have stipulated that patients must have an eGFR >30 mL/min/1.73m2, VICTORIA included patients with renal function as low as eGFR >15 mL/min/1.73m2.19 VICTORIA also enrolled a wider group of patients in terms of left ventricular ejection fraction inclusion criteria: <45% versus ≤40% for comparator HFrEF studies.6,7,19,27-33
Overall, this trial design yielded a different patient population compared to contemporary HFrEF trials, said Butler.6,7,19,27-33 Natriuretic peptide levels, a key marker of high risk, were substantially greater in the VICTORIA trial (median: 2,816 pg/mL) and the proportion of patients with NYHA Class III or IV symptoms (41%) was higher than in most other studies. Over one-half of the patients in VICTORIA trial (53%) had an eGFR <60 mL/min/1.73m2, indicative of chronic kidney disease (a higher rate of renal dysfunction than all other comparator studies). Overall, these clinical characteristics translated into a substantially higher risk patient population, explained Butler. This was evidenced by the fact VICTORIA had the shortest median follow-up time of all recent trials in HFrEF (10.8 months) because patients were so high risk that events were accrued quickly. Similarly, the primary endpoint event rate of first HFH or CV death in the control arm was “extraordinarily high” in VICTORIA, remarked Butler, and substantially greater than in most contemporary studies. Events per 100 patient-years were 37.8 in VICTORIA, compared to 13.2 in PARADIGM-HF (sacubitril/valsartan), 15.6 in DAPA-HF (dapagliflozin), 21.0 in EMPEROR-Reduced (empagliflozin), and 26.3 in GALATIC-HF (omecamtiv mecarbil).6,7,19,27-33
Butler emphasised that although the relative risk of the primary composite endpoint of CV death/HFH was only reduced by 10% in VICTORIA, because of the very high-risk nature of the population, this equated to a “substantial ARR of 4.2%.”19 Significant reductions in total HFH and the composite of all-cause mortality or HFH were also seen with vericiguat, and there was a directional benefit in the outcomes of CV death, HFH, and all-cause mortality.19 Although direct head-to-head comparisons between different agents have not been conducted, the ARR in VICTORIA, which Butler described as a ”measure that is very important to our patients,” was similar or numerically better than other recent trials in HFrEF. ARR for the primary endpoint was 4.2 in VICTORIA compared to 2.7 in PARADIGM-HF, 4.0 in DAPA-HF, and 5.2 in EMPEROR-Reduced.33
The clinical benefit of vericiguat was seen across all prespecified subgroups defined by the index event, including hospitalised patients, indicating a generally consistent treatment effect.19 The primary composite endpoint outcomes were also directionally consistent irrespective of sacubitril/valsartan use at baseline. This is important because an increasing proportion of HFrEF patients are on this background therapy, explained Butler.19
Regardless of patients’ baseline renal function, vericiguat provided benefit in clinical outcomes of HFH or CV death and HFH or all-cause death, with a trend towards reduced CV death also seen across all eGFR categories.21 The association between worsening renal function (WRF) and subsequent clinical outcomes was also explored in the VICTORIA trial and the benefit of vericiguat proved consistent even in subjects who developed WRF, with similar effects on the primary endpoint (interaction; p=0.76).21 Patients treated with vericiguat showed a stable pattern in terms of changes in eGFR and creatinine over time, with little fluctuation in renal function trajectories (Figure 3). This confirms not only the efficacy but also the safety profile of vericiguat in terms of renal function, noted Butler, and is important because cardiologists are particularly cognisant of changes in renal function, which can preclude optimisation of medical therapy, especially with renin-angiotensin-aldosterone system inhibitors (RAASi).
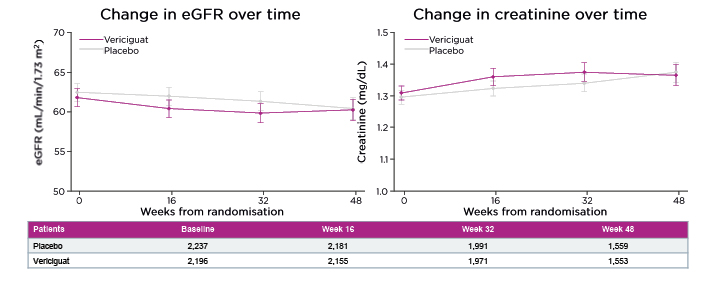
Figure 3: Impact of vericiguat on renal function trajectories was similar to placebo.²1
eGFR: estimated glomerular filtration rate.
Panel Discussion: Managing the Patient After a Worsening Heart Failure Event
Patients with worsening HF are commonly encountered in everyday clinical practice and constitute a cohort with significant unmet medical need. Jankowska reiterated that these patients are at very high risk; recurrent HFH events cannot be prevented despite life-saving foundational therapies, meaning additional treatment interventions are required. Overall, the panel agreed that discharge provides a “critical window” to maximise disease-modifying therapies for previously hospitalised patients with worsening HF and alter their disease natural history. Zannad suggested that the early 1–2-week post-discharge visit, recommended in the updated ESC guidelines, could also prove an opportune time to implement add-on therapy with vericiguat.
On the subject of comorbidities, Butler acknowledged that this is a complex and multidirectional issue in HFrEF. Renal function is particularly relevant because patients with worsening HF are at increased risk of WRF. VICTORIA was the first trial to enrol patients with an eGFR as low as 15 mL/min/1.73m2 and, with over 5,000 patients recruited, provided the power to look at several different subgroups. With vericiguat, the clinical benefit was accrued across the complete spectrum of eGFR at baseline, with negative p interaction values for all outcomes, explained Butler. The data on renal function trajectories with vericiguat also remained stable over time, even in these subjects with very low eGFR. Similarly, the overall number of patients who developed WRF was balanced between the two study arms and did not predict any lowering of vericiguat benefit. Results from VICTORIA also confirmed that the clinical benefits of vericiguat were retained across other key comorbidities including coronary disease with ischaemic and non-ischaemic aetiology and AF. Comorbidities are so common in patients with HFrEF that to have the signal for safety and efficacy with vericiguat ”is very important,” Butler concluded.
The panel then discussed what constitutes optimised GDMT. Butler emphasised the importance of exploring underlying reasons that may have precluded optimisation of a patient’s foundational therapies such as intolerance. VICTORIA shows that the clinical benefit of vericiguat is maintained “on top of optimised GDMT,” he added, as study participants were very well-treated at baseline (>90% RAASi and β-blocker use, 70%+ mineralocorticoid receptor antagonist use, and 60% on triple therapy). Vericiguat also boasts a further advantage, noted Butler. Of the four most common reasons why patients are unable to tolerate optimal medical therapy (increased heart rate, hyperkalaemia, hypotension, and increased creatine), none apply to vericiguat.
On the issue of safety, Lam emphasised that vericiguat was very well tolerated in the VICTORIA trial, with almost 90% of patients successfully uptitrated to the full 10 mg/day target dose. Mean BP was only a few mmHg lower in patients receiving vericiguat versus placebo and this effect manifested early, resolving within 4 months. VICTORIA also looked specifically at patients vulnerable to hypotension such as the elderly, those with lower baseline BP, and patients receiving angiotensin receptor-neprilysin inhibitors. In all these subgroups, there were no further increases in symptomatic hypotension or syncope episodes with vericiguat treatment.
The panel agreed that, based on resoundingly positive results from the large-scale VICTORIA trial, it was now time to focus on the clinical practice implementation of vericiguat. Zannad noted that this should be “very straightforward,” based on the robust comorbidity data and good safety profile. The EMA label specifies that patients must be stabilised before starting vericiguat and, in this context, Lam suggested stability would mean ensuring patients are decongested and ready to be discharged. Butler also added that ongoing clinical monitoring requirements for vericiguat should prove “easy” as there are no creatinine, potassium, or heart rate issues and only routine BP monitoring is recommended.
Summarising the key messages from the symposium, Zannad reiterated that patients with HFrEF remain at high risk of recurrent HFH and death after discharge. For these patients with worsening HF, the VICTORIA trial has shown the clear benefit of vericiguat in significantly reducing time to CV death or HFH, with an ARR in the range of contemporary HFrEF trials recently conducted. Vericiguat, therefore, marks an important new addition to the HFrEF armamentarium, concluded Zannad. Its implementation in clinical practice will help to optimise management and reduce the “enormous risk” faced by patients with worsening HF.








