Abstract
Psoriasis is a common skin disease with various cutaneous manifestations and is classified into two clinical groups: non-pustular and pustular. Pustular psoriasis is less common than non-pustular forms of psoriasis and is particularly resistant to treatment. Studies of the rarer variants of acrodermatitis continua of Hallopeau and pustular psoriasis of the tongue remain scant. The subtypes of psoriasis can present all over the body, including in uncommon locations, such as the oral cavity; however, there are limited presentations and data regarding oral involvement in psoriasis and its subsequent management. Although cases involving oral psoriasis are rare, with <100 publications in the literature, and generally asymptomatic, recent studies have suggested that it is more prevalent than once thought. In contrast, presentation and subsequent management of lingual pustular psoriasis have not been reported. Presented and discussed in this review is a rare case of symptomatic, painful lingual pustular psoriasis and acrodermatitis continua of Hallopeau with complete remission after the use of adalimumab, followed by a thorough review of the histopathology, diagnosis, and clinical management of oral psoriasis. The use of biologics for conditions involving the oral mucosa, particularly in the setting of cutaneous psoriasis, is a novel concept with potential application in the fields of dermatology, oral medicine, and rheumatology.
BACKGROUND
Psoriasis is a well-described dermatological disease with various cutaneous manifestations. The aetiology of this skin disorder is multifactorial, with a strong hereditary and genetic component, particularly regarding the PSORS1 gene and the human leukocyte antigen (HLA)-Cw6 allele.1 The subgroup of non-pustular psoriasis includes psoriasis vulgaris or chronic plaque, guttate psoriasis, erythrodermic psoriasis, palmoplantar psoriasis, inverse psoriasis, and psoriatic arthritis. The subgroups of pustular psoriasis are generalised pustular psoriasis, also known as von Zumbusch disease, and localised pustular psoriasis, which includes palmoplantar pustular psoriasis and acrodermatitis continua of Hallopeau (ACH), which targets the nailbeds and surrounding skin.1 Pustular psoriasis is particularly resistant to treatment. Several 2–3 mm sterile pustules that can easily rupture develop from an erythematous base and can coalesce into large pustular lesions. In extremely rare cases, pustules can develop on the oral cavity mucosa, lips, and lingual mucosa.2,3 Generalised pustular psoriasis is an uncommon, severe variant of pustular psoriasis. Some researchers believe generalised pustular psoriasis is a different inflammatory condition to generalised plaque psoriasis.2 There is limited literature regarding the efficacy of management of pustular psoriasis and a very small number of publications on rarer variants and their management, including ACH and pustular psoriasis of the tongue.4-6
Clinical and scholarly debates continue as to whether oral lesions in psoriasis are distinct pathological entities or whether they are indeed oral presentations of psoriasis.4,7 Oral psoriasis is described in the literature as temporary and generally asymptomatic, and is commonly referred to as geographic and fissured tongue (FT).4,8 Cases involving lingual psoriasis are exceptionally scant, with <100 publications available,6,9 and presentation and subsequent management of lingual pustular psoriasis have not yet been reported. Presented and discussed in this report is a rare case of symptomatic and painful lingual pustular psoriasis and ACH with complete remission following the use of adalimumab. A thorough review of oral psoriasis, including the histopathology and clinical management, is also included.
CASE REPORT
A 79-year-old Caucasian female presented with an 18-month history of recurrent painful oral lesions. She was diagnosed 3 years earlier with generalised pustular psoriasis (biopsy of the right thigh) and ACH (affecting left digits 1–3 and the right thumb, with ongoing nail involvement). Examination revealed dystrophic nails manifested by subungual hyperkeratosis and pustules, yellowish discolouration, and onycholysis, and the dorsal tongue was oedematous with widespread erythematous pustules (Figure 1). The rest of the body was unaffected. Her comorbidities included rheumatoid arthritis (RA), polycythaemia rubra vera (PRV, positive JAK2 mutation), iron deficiency anaemia, hypertension, and atrial fibrillation. Differential diagnoses of her clinical features included infectious causes (bacterial, fungal, viral), cellulitis, and herpetic whitlow.
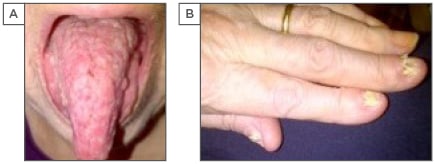
Figure 1: Psoriatic tongue (A) and nails (B) at presentation.
A) Extended tongue with pustular, erythematous lesions spread throughout the dorsal surface of the tongue and oral mucosa. B) Presentation of left hand with dystrophic nail changes on digits (thumb, index, and middle).
An oral surgeon performed a diagnostic incisional tongue biopsy. The dermatohistopathology revealed typical features of pustular psoriasis, similar to the report of her prior thigh biopsy (Figure 2). The absence of yeast or hyphae after staining the specimen with haematoxylin and eosin or Periodic acid–Schiff–diastase ruled out the presence of oral candida or fungal infection as causes of pathology. Topical swabs and viral screens were negative for unusual bacterial or viral growths.
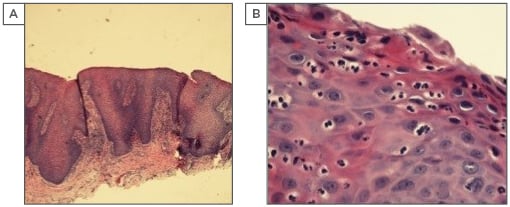
Figure 2: Histological specimens of the tongue biopsy with haematoxylin and eosin staining at 200x (A) and 400x (B) magnifications.
Histopathological features revealed subepithelial stroma with moderately dense perivascular and interstitial mixed inflammatory cell infiltrate consisting of lymphocytes and neutrophils, psoriasiform hyperplasia of the epithelium with suprapapillary plate thinning, and elongation of the rete ridges, acanthosis, and parakeratosis.1 Spongiotic pustules and subcorneal pustule were noted. The lamina propria showed elongation and thinning of the overlying epithelium. Dilated tortuous capillary loops were present in the dermal papillae.
Initial management provided temporary relief and consisted of xylocaine oral viscous, clobetasol deproponate (Dermovate®, GlaxoSmithKline, Uxbridge, UK), anti-calcineurin ointment (Protopic®, LeoPharma, Ballerup, Denmark), acitretin (Soriatane®, Stiefel, a GSK company, Research Triangle Park, North Carolina, USA) 10–30 mg/day, and mycostatin mouthwash. Later she retried methotrexate 12.5 mg/week for her oral psoriasis; however, it only provided a slight improvement and was subsequently stopped due to elevated liver enzymes. Prednisone 10 mg/day was given intermittently. Her mouth pain was interfering with eating, mastication, and deglutition, resulting in significant weight loss over several months (her BMI decreased from 21 to 18). None of these medications (including acitretin, methotrexate, and low-dose prednisolone) controlled her disease.
After consultations with her haematologists and discussions regarding the use of anti-TNF-α biologics, it was agreed with the patient to initiate adalimumab (Humira®, AbbVie, Ludwigshafen, Germany). An 80 mg loading dose of adalimumab infusion was given followed by a 40 mg subcutaneous injection every 2 weeks. Treatment with adalimumab was well-tolerated and effective. The psoriatic tongue lesions resulted in complete remission after 8 weeks and her nail involvement also cleared. Oral and additional arthritic symptoms were alleviated, which allowed the patient to start eating again, regain weight, and increase daily activities.
Although biologics are intended for long-term use in central psoriatic disease, they are not curative. The patient remained in remission for several years; however, in the spring of 2018, she developed congestive heart failure and adalimumab was stopped. Acitretin 10 mg was started during remission. After 3 months on acitretin, her oral psoriasis and ACH started to flare and she became symptomatic. Adalimumab will likely be restarted in the future because the patient is no longer experiencing congestive heart failure and had a good response when the drug was used previously.
DISCUSSION
Review of Oral Lesions in Psoriasis
Oral psoriasis is a collective term for lesions presenting anywhere in the mouth, including the lips, tongue, buccal mucosa, palate, and gingivae.10-12 Van der Waal and Pindborg13 classified oral psoriasis (Table 1a), with further sub-categorisation described by Younai and Phelan,9 consisting of two major types and five minor subtypes (Table 1b). Younai and Phelan9 identified 57 cases of oral mucositis with similar histology to psoriasis, which appeared in various anatomical regions, including the oral cavity, buccal mucosa, tongue, gingiva, palate, floor of mouth, and the vermillion border of the lip. Although this report does not seemingly correspond with the criteria of oral psoriasis, the diagnosis of oral psoriasis should not be immediately excluded.
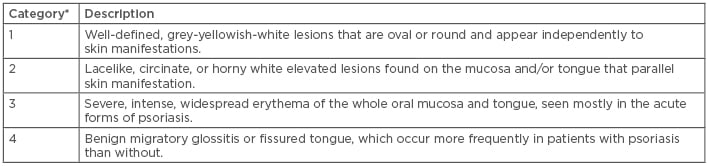
Table 1a: Classification of oral psoriasis.13
* Types 1, 2, 3, and 4 have progressive signs and symptoms in each category. Overlaps may occur.
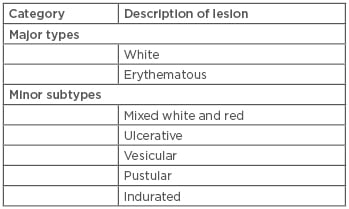
Table 1b: Sub-categorisation of oral psoriasis.4
The involvement of the oral mucosa in psoriasis remains a controversial subject in the field and is thought to be uncommon and infrequent. However, Talaee et al.14 demonstrated that the prevalence of oral involvement in psoriasis is common, occurring in 47% of patients (83% of these cases were generalised plaque psoriasis), and involvement of the oral mucosa is significantly associated with younger age (21–30 years), previous history of oral lesions, and an early disease onset. This may suggest oral involvement in psoriasis is under-reported in the literature. There were no cases of pustular psoriasis or pustules on the oral mucosa in the Talaee et al. study,14 similar to the case presented in this report.
The occurrence of isolated oral psoriatic lesions without cutaneous manifestations is rare because oral involvement is more common in patients with severe forms of cutaneous psoriasis, particularly generalised plaque and pustular psoriasis.1 There have been occasional reports of isolated oral psoriasis without cutaneous psoriasis; in a series of cases, ˜50% occurred with skin psoriasis, in ˜10% oral psoriasis preceded the onset of skin psoriasis, and in ˜20% cases of oral psoriasis were isolated.8,10,11
The most common location for oral psoriatic lesions is the tongue4,8-10,12 and these lesions can be categorised into two major groups. One group includes mucosal abnormalities with corresponding psoriasis-like histology and often parallels the clinical course of cutaneous psoriasis, as seen in this case. The second category is more common and comprises a range of nonspecific lesions, such as benign migratory glossitis (BMG) and FT, that are thought to occur more often in patients with typical cutaneous psoriasis forms.12,15,16 A previous literature review demonstrated that the prevalence of FT ranges from 9.8–48.5% and BMG ranges from 5.6–18.2% in psoriatic patients, with most cases commonly presenting in patients with plaque psoriasis.17 There remains minimal description of oral mucosal presentation and involvement in pustular psoriasis cases in the clinical literature.4,8-10,12,14,15,17
BMG (also termed geographic tongue, migratory stomatitis/glossitis, annulus migrans, stomatitis areata migrans, erythema circinata, geographic stomatitis, and ectopic geographic tongue) is described as >1 sharply demarcated erythematous patch with raised white or yellow serpiginous borders, the colour and shape of which will change over time.15 Migratory lesions change location and prominence daily and tend to occur during flares of psoriasis.15 BMG is an inflammatory disorder of unknown aetiology (similar to psoriasis) and is generally described as asymptomatic with a psoriasiform mucositis of the dorsum of the tongue affecting the epithelium. This results in ulcerative lesions from loss of local filiform papillae surrounded by white lines.15 Abe et al.18 and Ishibashi et al.19 reported three cases of long-standing symptomatic and painful BMG. BMG is thought to present similarly to psoriasis in regard to genetic and histopathological aspects and also has similar clinical features.20 Accordingly, rather than being considered as an entity on its own, many clinicians and researchers consider BMG as an oral manifestation of psoriasis. Current publications on BMG are limited and the topic remains controversial as to whether BMG is a particular oral form of psoriasis or a clinical condition on its own.17 Nevertheless, several studies have reported significant links between the prevalence of BMG in psoriatic patients, particularly in patients with more severe forms of psoriasis.14,17,20
FT (also termed lingua fissurata, lingua plicata, scrotal tongue, grooved tongue) is another common form of oral presentation in psoriasis. It is recognised clinically by an anteroposterior groove on the dorsal tongue, often with lateral extending branching fissures.16 Darwazeh and Almelaih21 showed that 23% of patients with FT experienced painful symptoms, particularly when eating.
BMG, FT, and generalised pustular psoriasis are three disorders that have polygenic inheritance patterns and it is feasible that affected patients may share genes for these conditions.8,10 Several authors have claimed that BMG is more prevalent in patients with generalised pustular psoriasis and is associated with severity of disease.15,16 Others dispute that FT is more prevalent than BMG in patients with generalised pustular psoriasis and assert that with increasing age of onset and severity of psoriasis (assessed by Psoriasis Area Severity Index [PASI] scores), FT occurs more often in generalised pustular psoriasis, while BMG incidence increases with disease severity in generalised plaque psoriasis.12,15,16
While the debate regarding oral involvement is ongoing, the presentations of oral lesions in psoriasis are generally described as asymptomatic or temporary.9 The case presented in this report demonstrates a rare form of oral psoriasis, with an erythematous, oedematous, and pustular tongue that did not present clinically like BMG or FT and was refractory to various treatments. Also, there was debilitating long-standing pain involvement, which is an unusual and perhaps a new noteworthy feature for oral psoriasis.
ACH is a rare variant of pustular psoriasis that involves the nails and nailbeds of the fingers and toes, resulting in painful nail dystrophy and paronychial erythema.22 ACH is linked with inflammatory arthritis and generalised pustular psoriasis.23 Although rare, ACH is also associated with pustules occurring in the oral mucosa (particularly the tongue), conjunctiva, and urethra, and is distinct from Reiter’s syndrome and Behçet’s syndrome.22,23
Diagnosis of Oral Psoriasis
The diagnosis of oral psoriasis is most accurate when the clinical oral presentations parallel those of cutaneous lesions and are supported by histological findings from a biopsy. Criteria for clinical diagnosis solely have been suggested, including a positive family history for psoriasis, oral lesions that parallel the clinical course of skin manifestations, HLA typing (commonly for B13, B17, B37, Cw4, and Cw6), and exclusion of other causes.1,11,24 Studies have shown that HLA-Cw6 correlates with generalised plaque psoriasis, while BMG correlates with HLA-B15 and DR7 and FT is associated with HLA-DRB1.1-12,14-25
It is important to consider differential diagnoses in oral mucosal conditions, including malignancy, oral candidiasis, lichen planus, secondary syphilis, systemic lupus erythematosus, pemphigoid (bullous or cicatricial), pemphigus (vulgaris), leukoplakia, Behçet’s syndrome, and Reiter’s syndrome.11,22,23 Since these entities have distinct histopathological criteria, clinical presentation along with biopsy is fundamental in confirming diagnoses of oral psoriasis by effectively ruling out other diagnostic entities. In the present case, while the patient did have arthritis, the other symptoms and histopathological features typical of the Reiter’s syndrome triad were absent.22 Generally, oral psoriasis pathology has similar histopathologic features to cutaneous psoriasis. Yet, clinical changes in the tongue are often nonspecific and histological correlation is helpful for confirming diagnosis.4
Histological findings of oral psoriasis comprise hyperkeratosis or parakeratosis, elongation and clubbing of rete ridges, and thinning of the epithelium superior to the dermal papilla. Infiltrates of inflammatory cells are prevalent and also reflect the stage of the lesion (leukocytes are a sign of early stage while lymphocytes signify later stages). Munro’s microabscesses and spongiform pustules of Kogoj rarely occur in oral psoriasis compared to cutaneous psoriasis but may occur in early lesions.11 Psoriasiform mucositis is nonspecific for other conditions (such as Reiter’s syndrome).22,24 Negative scrapings for excessive candida and repeated microbial swabs ruled out fungal or infective causes of pathology. Other differential clinical entities, such as oral lichen planus and lichenoid reactions, have distinct histological and clinical features and were not seen in this case. Accordingly, histopathology together with clinical presentation and investigation are essential to support a subsequent diagnosis.22
Treatment and Management of Oral Psoriasis and Acrodermatitis Continua of Hallopeau
Literature detailing clinical treatments for the rarer forms of psoriasis remain limited and need further investigation and guidance.25,26 Management of oral psoriasis and other inflammatory conditions involving the oral mucosa are mostly based on case reports and off-label uses of biologics and systemic immunotherapy.26 Currently, there are no reports of managing pustular psoriasis of the tongue. Discussed below are suggested measures used in the management of this case, which may be suitable for future applications in dermatology and other medical or dental specialities.
Topical and Conservative Measures
Suggested conservative measures that are quick and feasible include removal of irritants and infection and managing existing orodental pathology. Simultaneous candidiasis can complicate diagnosis and management and may be successfully treated with oral antifungals or mycostatin mouthwash.20 Physical manipulations should be minimal and performed with caution because there may be a potential Koebnerization effect on the inflamed mucosal tissue.
Oral psoriatic lesions are usually temporary and asymptomatic, yet this patient experienced chronic painful lesions. Palliation with a topical anaesthetic, including viscous lidocaine or diphenhydramine, mucosal protectants (Orabase®, ConvaTec Inc., Reading, UK) or magnesium and aluminium hydroxides (Maalox®, Sanofi, Origgio, Italy), and alkaline rinses can be used to minimise painful discomfort. Topical corticosteroids, such as fluocinonide gel 0.05% (Lidex®, County Line Pharmaceuticals, Brookfield, Wisconsin, USA), may be applied for symptomatic relief.20
Systemic and Immunomodulating Therapies
Retinoids such as acitretin are often used as first-line systemic therapy in males and non-fertile females with psoriasis.5 Case reports have shown successful management of ACH with acitretin in combination with topical calcipotriol.5 However, the present case showed no nail or lingual improvement with acitretin.
Systemic use of the immunomodulator methotrexate is a recognised treatment for psoriasis, particularly severe and refractory cutaneous plaque psoriasis, and has been used for pustular psoriasis. It is also licensed for use in several other chronic and refractory inflammatory conditions, e.g., RA, ulcerative colitis, and malignancies.27 Systemic use of methotrexate is often not tolerated by patients and carries inevitable risks with long-term use (e.g., pancytopenia, hepatotoxicity).27,28 Methotrexate targets cells undergoing rapid turnover, such as those in the mucosa and bone marrow, often causing mucositis. Accordingly, methotrexate is not routinely used for oral inflammatory lesions because a common adverse reaction is oral ulceration.28 While methotrexate provided some relief to our patient’s nail symptoms, it did not resolve her oral symptoms. Calcineurin inhibitors (cyclosporine A and tacrolimus) are rapid and effective treatments for cutaneous psoriasis. They are useful for treating generalised pustular psoriasis and oral lesions, including gingival hypertrophy, mouth sores, swallowing difficulty, gingivitis, gum hyperplasia, xerostomia, abnormal taste, tongue disorder, and gingival bleeding.24 Abe et al.18 described the successful treatment of painful geographic tongue in a 54-year-old woman using systemic cyclosporine, while Ishibashi et al.19 described two patients aged 77 years with symptomatic migratory glossitis that were successfully treated with 0.1% topical tacrolimus.
Often, immunomodulator drugs are used in combination with systemic retinoids, oral corticosteroids, and light therapy, which can generate satisfactory results.29 Yet, with time, relapses and a lack of efficacy occur. Immunomodulators are known to become refractory, often not tolerated by patients, and carry inevitable risks with long-term use (e.g., pancytopenia, hepatotoxicity, nephrotoxicity).
Hydroxyurea is an older treatment once used for pustular psoriasis. Currently, it is not indicated or commonly used in practice for this condition;30 however, hydroxyurea is clinically indicated for treating PRV, which this patient had as a comorbidity.30 Interestingly, the patient started hydroxyurea for PRV, which likely had some mild additional benefit for her cutaneous and mucosal pustular psoriasis.
Biologic Therapies
TNF-α is a well-studied cytokine involved in the pathogenesis of psoriasis; specifically, it increases immune cell infiltration to the skin causing keratinocyte proliferation.1,31 There are three main anti-TNF-α biologic drugs licensed for psoriasis and inflammatory arthropathies: two recombinant monoclonal antibodies that target TNF-α directly, adalimumab (Humira) and infliximab (Remicade®, Janssen, Leiden, Netherlands), and a fusion protein, etanercept (Enbrel®, Pfizer, Sandwich, UK), which antagonises the TNF-α receptor.26,31 These biologics are licensed for severe refractory patients with inflammatory conditions, notably psoriasis, Crohn’s disease, ulcerative colitis, RA, and systemic lupus erythematosus.31 There are several off-label uses of these drugs for conditions with similar pathogenesis involving TNF-α, including ACH and various mucosal conditions.26,32 The literature reports successful off-label use of biologics in patients with mucosal conditions, including Behçet’s syndrome, recurrent aphthous stomatitis and ulcers, benign mucous membrane pemphigoid, and lichen planus.26,33
Few case reports exist of the use of biologic therapies for the targeted management of oral psoriasis; however, off-label use in inflammatory oral mucosal conditions is evident. Infliximab is a well-established biologic used in psoriasis vulgaris and psoriatic arthritis that has been shown to have the longest rates of efficacy and patient retention when compared with other anti-TNF biologics.26 Connolly et al.34 described the successful treatment of Behçet’s syndrome in a young woman with a 30-year history of orogenital ulcerations using infliximab therapy.34 A good initial response with infliximab for treating severe pustular psoriasis and ACH was shown by Newland et al.;35 however, there was subsequent unresponsiveness after 18 months.
Adalimumab has been licensed for and used successfully in cutaneous psoriasis, including both generalised plaque psoriasis and pustular psoriasis.32 Chao36 described the success of adalimumab in treating cutaneous and oral lichen planus. For the present case, adalimumab was chosen after considering the patient’s comorbidities and convenience. After 8 weeks of adalimumab, complete remission of lingual pustular psoriasis was achieved, with added amelioration of ACH and inflammatory arthritis.
Other biologics for treating psoriasis exist. Secukinumab is a human Ig monoclonal antibody that targets and neutralises the inflammatory cytokine IL-17A, which has been found to induce transcription of other proinflammatory cytokines, chemokines, and effectors in inflammatory conditions like psoriasis.37 A recent case report in 2017 of a 42-year-old female patient with a similar presentation of ACH and painful erosive oral mucositis of the tongue and palate causing pain and weight loss was described by Baron,6 and successful use of secukinumab induced remission.6 Adalimumab, cyclosporine, and methotrexate were considered possible options but secukinumab was found to better suit their patient. In Baron’s case, secukinumab was effective at responding to the patient’s atypical disease and symptoms.6 At the time of the case described in the present report, secukinumab was not readily available, but now may be considered an option for future treatment.
CONCLUSION
Here the authors report the successful use of adalimumab for inducing remission of a rare presentation of symptomatic pustular oral psoriasis. This case represents interesting novel signs and symptoms of oral pustular psoriasis and describes the rationale for management deriving from challenging comorbidities. Oral psoriasis is typically described as asymptomatic but, as shown in this case, can be painful and debilitating. It should be considered in the differential diagnosis of painful lesions of the oral mucosa that are not responding to usual therapies, especially in the setting of psoriasis. Biopsies are helpful for diagnosis and can influence management plans; however, treatment remains challenging since few reports of successful therapies exist for such presentation.
Accordingly, the use of adalimumab in this case highlights a potential management strategy and the treatment of a complex dermatological case that overlaps with conditions from other medical specialities. The exact aetiology, pathogenesis, and optimal management of oral psoriasis, particularly pustular lingual psoriasis, remains obstinately obscure. More research, reporting, and biomedical investigations into this disease and its progression are needed. Furthermore, clinical suspicion should lead dermatology, rheumatology, immunology, and oral medicine and pathology specialists to consider the use of biologic anti-inflammatory therapies to relieve patients of disease progression and discomfort.







