Meeting Summary
This symposium took place at the 27th European Academy of Dermatology and Venereology (EADV) Congress. The session examined the latest data for contemporary therapeutic agents in psoriasis, focussing on IL-23 inhibitors as the most recently approved class of therapies, and provided perspectives on the implications of these data for clinical practice. With a wide array of potential treatment options now available for psoriasis, the symposium initially explored remaining areas of unmet treatment need, highlighting correct and timely diagnosis, effective management of comorbidities, undertreatment, and real-world data as key aspects requiring further improvement. The speakers subsequently reviewed the current evidence for the latest therapeutic strategies in psoriasis, concentrating on the therapeutic attributes that are considered most desirable for an ‘ideal’ agent, including efficacy for psoriasis and related comorbidities, durability of effect, improvement in quality of life, safety, and convenience. In this context, the rationale for selective IL-23 inhibition was examined, with the faculty highlighting how this approach differs from IL-17 inhibitors, at both the mechanistic and clinical levels. In addition, the session called attention to areas of ongoing investigation where there may be opportunities for the latest therapies to provide further patient benefit, with focus on the potential for novel, less frequent dosing intervals with IL-23 inhibitors.
Introduction
Professor Kristian Reich
Despite recent advances, there remains substantial unmet need in the treatment of psoriasis and further progress is required. IL-23 inhibitors represent the latest class of therapies to emerge, adding to already available agents, which include TNF inhibitors, IL-12/23 inhibitors, and IL-17 inhibitors. Given the spectrum of potential treatment options available, it is important to understand the role and importance of each class of agent in the therapeutic armamentarium.
Are There Still Unmet Needs in the Evolving Psoriasis Treatment Landscape?
Professor Richard Warren
Psoriasis is a serious global problem, as acknowledged by the World Health Organization (WHO) in their recent Global Report on Psoriasis, issued in 2016.1 Worldwide, 125 million patients are affected by psoriasis,2 approximately 14 million of whom reside in Europe.3 Key areas of unmet medical needs in psoriasis relate to correct and timely diagnosis, effective management of comorbidities, addressing undertreatment, overcoming the challenges posed by psoriasis occurring in difficult-to-treat areas, and the lack of real-world patient data with newer therapeutic agents.1,4-6
Improving the management of psoriasis requires early diagnosis, timely referral, and correct assessment of disease severity.1 Patient and physician perceptions of psoriasis severity may differ,1 as illustrated by evidence from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey.4 In the MAPP survey, 22% of patients who had ≤3 palm lesions considered their psoriasis to be severe,4 which is likely to differ from the physician-perceived severity of such cases of psoriasis. The lack of concordance between patient and physician-perceived severity indicates a need for improved methods for assessing severity in the clinic. Beyond the severity of psoriasis, it is also important to consider the presence of comorbidities when selecting an appropriate therapeutic strategy. Psoriatic arthritis, hypertension, depression, Type 2 diabetes mellitus, obesity, and hyperlipidaemia are all common comorbidities in patients with psoriasis.7,8 In addition, Crohn’s disease is genetically linked with psoriasis and represents a further potential comorbidity.9 Taken together, the physical and psychological impact of psoriasis and associated comorbidities may have a cumulative impact on patients’ lives over time, particularly for those patients who are less adept at coping with their condition, ultimately altering patients’ life choices and impacting the course of their lives.10,11 This concept is known as ‘cumulative life-course impairment’ and highlights a need for early and effective treatment of psoriasis and related comorbidities.10,11
With regard to treatment standards and the unmet need in psoriasis, the recently conducted ‘Clear About Psoriasis’ survey of >8,000 patients with moderate-to-severe psoriasis from 31 countries indicated that a large number of patients remain dissatisfied with their psoriasis treatment.12 Within this study, 57% of patients reported having not achieved clear or almost clear skin with their current treatment regimen.12 While 56% of patients reported that they were ‘satisfied’ with their treatment, 24% were ‘uncertain’ and 20% were ‘dissatisfied’, with the majority of dissatisfied patients (89%) not achieving clear or almost clear skin.12 Such dissatisfaction may be linked with undertreatment; in the MAPP survey, nearly 40% of patients with >10 palm lesions were receiving no treatment, and only 11% of those patients were receiving oral or biologic therapy.4 Among the audience members at this symposium, the majority considered undertreatment to be a bigger unmet need for patients with psoriasis than delayed (or incorrect) diagnosis. The challenge of undertreatment may be related to the high proportion of patients who are affected by psoriasis in difficult-to-treat areas, such as the scalp, face, nails, genitals, intertriginous areas, palms, and soles.6 These psoriasis subtypes may disproportionately impact patients’ quality of life, while simultaneously not meeting the criteria for access to the most effective therapies if assessed using thresholds such as body surface area affected of >10%, leading to undertreatment.6 Furthermore, treatment of such subtypes may require a tailored therapeutic strategy, as agents commonly used for psoriasis are not always suitable or effective in treating psoriasis affecting these specific areas.6
Over 70% of attendees at the symposium indicated that long-term real-world data have greater influence on their prescribing decisions than robust Phase III data from clinical trials. The representativeness of clinical trials to real-world clinical practice is therefore key and has been explored in several analyses.5,13 In the UK, when data from the British Association of Dermatologists Biologic Interventions Register (BADBIR) registry were analysed, it was found that just over half (53%) of patients were considered to meet the enrolment criteria for the Phase III licensing studies for etanercept, adalimumab, or ustekinumab.5 Around one-third of patients (32%) had insufficient baseline data to allow analysis or missing data, and the remainder were considered ineligible (15%).5 Among the ineligible group, there were more elderly patients (aged ≥70 years) than in the eligible group and patients tended to have higher BMI, more comorbidities, and experienced smaller reductions in Psoriasis Area Severity Index (PASI) with treatment.5 Crucially, a higher rate of serious adverse events was observed in the ineligible patient group when treated with etanercept, adalimumab, or ustekinumab than in those patients considered eligible for the clinical trials.5 When interpreting clinical trial results, it is therefore critical to consider how representative the trial is of the real-world patient population; there is a need to improve under-representation of real-world patient subsets within clinical studies.
In summary, there are still numerous unmet medical needs affecting patients with psoriasis. Future efforts need to focus on encouraging earlier diagnosis of psoriasis and associated comorbidities, curtailing undertreatment, and addressing the under-representation of real-world patient subsets in clinical studies.
What is the Best Target for Psoriasis: IL-23 Versus IL-17A?
Doctor Andrew Blauvelt
While methotrexate and phototherapy formed the backbone of early management of psoriasis, recent decades have seen revolutionary changes in treatment, first with the emergence of TNF inhibitors, and more recently with IL-12/23, IL-17, and IL-23 inhibitors.14 The emergence of each class of new treatment option has reflected an evolving understanding of the pathogenesis of psoriasis, which is now understood to be primarily an immunologic disease mediated by dysfunction in regulation of the IL-23/Th17 axis.15-17 A key benefit of specifically targeting the IL-23/Th17 pathway is that although the pathway is involved in mucocutaneous immune defences,18 it is not involved in systemic immunity.19
Modern treatment options provide the opportunity to inhibit this pathway at various stages, including at upstream (e.g., IL-23 inhibitors), intermediate (e.g., IL-17 inhibitors), or downstream points (e.g., IL-17 receptor antagonists).16,17 Physicians are now faced with the challenge of determining whether to select an inhibitor targeting IL-23 or IL-17 as the therapeutic strategy for their patients.
Focussing first on treatment efficacy, primary endpoint data from pivotal clinical trials in moderate-to-severe psoriasis with biologic agents targeting IL-17 indicated PASI 75 response rates of 77–82% at Week 12 with the IL-17A inhibitor secukinumab (300 mg),20 87–90% at Week 12 with the IL-17A inhibitor ixekizumab (80 mg; every 2 weeks),21 and 85–86% at Week 12 for the IL-17 receptor agonist brodalumab (210 mg; every 2 weeks).22 In similar studies with IL-23 inhibitors, PASI 75 response rates of 61–64% were observed at Week 12 with tildrakizumab (100 mg),23 with rates of 86–91% seen at Week 16 with guselkumab (100 mg).24,25 Although the current lack of head-to-head clinical trials between IL-17 and IL-23 inhibitors limits the possibility of drawing robust conclusions about the comparative efficacy of these agents, ongoing studies are being conducted to address this question, including the ECLIPSE study,26 which will directly compare the efficacy of guselkumab with secukinumab.
Given the chronic nature of psoriasis, it is important that therapeutic agents have durable efficacy. Sustained PASI response rates over time have been demonstrated with up to 5 years’ treatment with secukinumab,27 with up to 3 years’ treatment with ixekizumab,28 and with up to 2 years’ treatment with guselkumab.29 In addition, it is interesting to note that the efficacy of guselkumab appears to be sustained for a substantial duration of time after withdrawal of therapy.25 In the VOYAGE 2 study,25 patients who had received 28 weeks’ guselkumab treatment and achieved PASI 90 were randomised to continued guselkumab therapy or withdrawal (placebo). Although PASI 90 and Investigator’s Global Assessment 0 or 1 (cleared or minimal) response rates at Week 48 were significantly greater in those receiving continued guselkumab therapy versus those who were withdrawn from therapy (p<0.001), 37% of patients in the withdrawal arm still had a PASI 90 response at Week 48 (28 weeks after the last guselkumab dose), and >40% had Investigator’s Global Assessment 0 or 1 responses (Figure 1).25
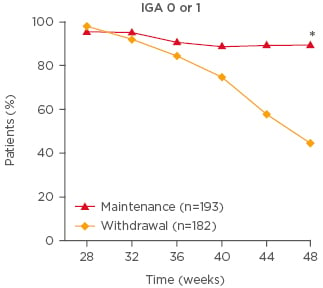
Figure 1: Investigator’s Global Assessment 0 or 1 response rate among patients withdrawn from or maintaining guselkumab therapy following an initial response† in the VOYAGE 2 trial.
*p<0.001; †≥90% improvement in Psoriasis Area Severity Index after 28 weeks’ guselkumab treatment.
Adapted from Reich et al.25
A previous study has explored the potential for prolonged efficacy to enable dosing-interval extension using the IL-12/23 inhibitor ustekinumab.30 In this study, patients with moderate-to-severe psoriasis responding (Physician’s Global Assessment [PGA] of 0 or 1) to 28 weeks’ ustekinumab treatment were randomised to either dosing every 12 weeks (in line with the recommended dosing regimen) or to a response-based dosing regimen, with a variable dosing interval ranging from every 12 weeks for those who lost response at Week 32 to every 24 weeks for those who maintained response at Week 40.30 This study found that in some patients, dosing can successfully be extended to every 6 months, with higher PGA 0 or 1, PASI 75, and PASI 90 response rates observed from Week 40–112 in patients in the subgroup who received 24-week dosing from Week 40 compared with those receiving more frequent dosing.30 Taken together, the results of the these studies of IL-12/23 inhibition with ustekinumab and selective IL-23 inhibition with guselkumab suggest that upstream inhibition of the IL-23/Th17 axis may be linked with sustained pharmacodynamic effects after the drug has been eliminated from the body. Given that Th17 cells are known to be dependent on IL-23 for cell survival, this result may indicate that IL-23 inhibition leads to death of pathogenic skin-resident memory Th17 cells, potentially leading to more prolonged disease control.31
Psoriatic arthritis is prevalent among patients with psoriasis,7 and it is therefore important to consider the efficacy of potential psoriasis treatment options on this comorbidity. Both secukinumab and ixekizumab have been approved in the European Union (EU) and the USA for the treatment of psoriatic arthritis.32-35 In Phase III trials in patients with psoriatic arthritis, these IL-17 inhibitors have been shown to significantly improve American College of Rheumatology (ACR) 20 response rates compared with placebo over 24 weeks.36-39 With regard to the efficacy of IL-23 inhibitors in patients with psoriatic arthritis, Phase II data have recently been published for guselkumab that showed significantly greater ACR 20 response rates at Week 24 versus placebo,40 with similar response rates to those seen in the previous studies with IL-17 inhibitors. These encouraging early data for guselkumab require verification in larger Phase III studies, which are currently ongoing.41,42
Safety is a critical factor when evaluating potential treatment options for psoriasis, given a likely need for long-term treatment. Agents directly targeting IL-17 or its receptor (e.g., secukinumab, ixekizumab, and brodalumab) are considered to be generally well-tolerated;43 however, consistent with the known role of the IL-17 pathway in resistance to mucocutaneous infections, such agents are associated with mucocutaneous candidiasis infections.32,33,44 In addition, exacerbations of Crohn’s disease have been seen in clinical studies with secukinumab,32 and cases of new onset or exacerbated Crohn’s disease and ulcerative colitis have been reported with ixekizumab.33 It has been hypothesised that IL-17 may play a protective role in the gastrointestinal tract, and therefore IL-17 inhibition may block this protective action, predisposing some patients to the development or exacerbation of inflammatory bowel diseases.45 Agents inhibiting IL-23 (e.g., ustekinumab, guselkumab, and tildrakizumab) are also considered to be generally well-tolerated43 but have not been reported to be associated with candidiasis or inflammatory bowel disease.46-48 Furthermore, ustekinumab is in fact indicated for the treatment of Crohn’s disease.47 In this context, it is important to note that not all IL-17A-producing cells are regulated by IL-23, including in the gut.49 These IL-23-independent pathways may allow for continued protective IL-17A production during IL-23 inhibition.49
An additional consideration when selecting the therapeutic regimen for psoriasis is the required frequency of dosing, which is an aspect in which IL-17 and IL-23 inhibitors differ. While IL-17 inhibitors require dosing every 2–4 weeks,32,33,44 IL-12/23 and IL-23 inhibitors are dosed less frequently, typically every 8–12 weeks.46-48
In summary, while IL-17 and IL-23 inhibitors both represent highly efficacious and broadly well-tolerated classes of therapy for psoriasis,43 differences exist between agents in durability, safety, and posology. It is also important to acknowledge that the therapeutic profiles of individual agents within each class may differ, likely driven by differences in antibody binding affinity, dose, dosing frequency, or other attributes.
Are We Thinking Long Enough? Applying Clinical Evidence to Practice
Professor Kristian Reich
Plaque-type psoriasis is driven by the interaction between the immune system and the epidermis. In the initial ‘feed-forward’ response, dendritic cells activate T cells via IL-23 release, which in turn release mediators, such as IL-17, that activate keratinocytes and stimulate keratinocyte proliferation, ultimately leading to psoriatic plaque formation.16 Once keratinocytes are activated, they release further mediators that signal back to the immune system, such as IL-8 which attracts neutrophils to the skin,16 creating a vicious circle with both feed-forward and feed-back responses between the immune system and skin.
In clinical studies in patients with psoriasis, high response rates have been observed with IL-17A inhibitors. With secukinumab, an average PASI 90 response rate of 75% was observed after 24 weeks’ treatment across the FIXTURE, CLEAR, and PRIME clinical studies, and a similar proportion of patients (75%) achieved absolute PASI scores ≤2.50 Response rates at Week 24 with secukinumab in these studies were higher than those seen with etanercept (PASI 90: 40%; PASI ≤2: 38%) or ustekinumab (PASI 90: 61%; PASI ≤2: 61%).50 Similarly, ixekizumab has demonstrated greater clinical efficacy in terms of PASI 90 and PASI ≤2 response rates at Week 24 (83% and 84%, respectively) compared with ustekinumab (59% and 62%, respectively; p<0.01).51 Taken together, these data suggest that IL-17A inhibitors provide greater response rates than ustekinumab. Ustekinumab is a monoclonal antibody that binds to the p40 subunit common to both IL-12 and IL-23, thereby inhibiting receptor binding and suppressing both the IL-12-mediated Th1 pathway and the IL-23-mediated Th17 pathway.47 In contrast, the IL-23-specific inhibitors, such as guselkumab, bind to the p19 subunit of IL-23, providing the opportunity for selective blockade of IL-23-mediated pathways.16,46
Pivotal clinical studies of guselkumab in patients with psoriasis include the VOYAGE 1 and 2 trials.24,25 In VOYAGE 1, patients receiving guselkumab achieved a PASI 90 response rate of 80% after 24 weeks’ treatment, with superior response rates to adalimumab (53%; p<0.001) (Figure 2).24 Notably, at the end of the 1-year study, almost half (47%) of patients in the guselkumab group achieved PASI 100, indicating clearance of psoriasis, compared with 23% of adalimumab-treated patients (p<0.001).24
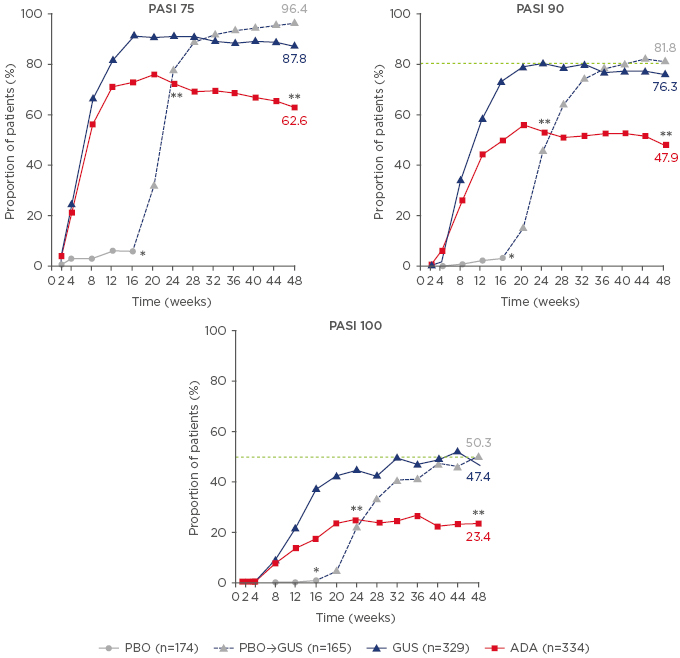
Figure 2: Psoriasis Area Severity Index response rates over time with placebo, guselkumab, and adalimumab in the VOYAGE 1 trial.
Data are from a non-responder imputation analysis. Patients in the placebo group switched to guselkumab treatment from Week 16 onwards.
*p<0.001 for GUS versus PBO; **p<0.001 for GUS versus ADA.
ADA: adalimumab; GUS: guselkumab; PASI: Psoriasis Area Severity Index; PBO: placebo.
Adapted from Blauvelt et al.24
The high rate of complete resolution of psoriasis with guselkumab may be important in the context of durability of efficacy and potential extension of the dosing interval, particularly given that a previous study with ustekinumab identified achievement of PGA 0 (cleared disease) as a predictor of ability to successfully extend the dosing interval while maintaining response.30 As mentioned in the previous presentation, VOYAGE 2 explored the efficacy of guselkumab after withdrawal, with patients responding to 28 weeks’ guselkumab therapy randomised to either withdrawal of therapy or continued guselkumab.25 In those patients withdrawn from guselkumab, the estimated median time to loss of PASI 90 response was >3 months (15 weeks).25 However, this evidence alone does not imply that patients with well-controlled psoriasis achieving PASI 90 with guselkumab can be withdrawn from therapy or switched to less frequent dosing in clinical practice; further data are required.
As highlighted earlier, many patients present with psoriasis involving the nails, hands, or feet.6 In the VOYAGE 2 study, among the subgroup of patients with hand/foot (hf) psoriasis, 77% of guselkumab-treated patients achieved a hf-PGA of 0 or 1 with a ≥2-grade improvement at Week 16, a significantly greater proportion than those receiving placebo (14%; p<0.001) and numerically more than those receiving adalimumab (71.4%).25,52 At Week 24, a significantly greater proportion of patients in the guselkumab group achieved the hf-PGA endpoint (82%) compared with adalimumab (66%; p=0.046),25,52 consistent with the previously discussed superiority of guselkumab over adalimumab for plaque psoriasis. In contrast, in those patients with fingernail involvement, no significant difference was seen between guselkumab and adalimumab in fingernail-PGA 0 or 1 response rates, which were significantly greater with guselkumab versus placebo at Week 16 (52% versus 15%, respectively) but not significantly different versus adalimumab at Week 24 (63% versus 67%, respectively; p=0.376).52 These results may indicate that the pathogenic contribution of TNF-α and IL-23 varies between different subtypes of psoriasis.
Given the impact of psoriasis on patients’ daily lives, including their psychological wellbeing, it is important to evaluate the effectiveness of treatment on patient-reported outcomes. In VOYAGE 2, among those patients with Hospital Anxiety and Depression Scale (HADS) scores indicating anxiety (HADS-A ≥8) or depression (HADS-D ≥8) at baseline, guselkumab was associated with greater improvements in anxiety and depression compared with adalimumab, as indicated by higher rates of patients achieving HADS-A <8 (58% versus 43%, respectively; p=0.028) or HADS-D <8 (60% and 46%, respectively; p=0.079).53 Improvements in anxiety and depression were correlated with improvements in psoriasis (assessed via PASI scores).53 More broadly, the clinical benefits of guselkumab appear to translate into improvements in quality of life, with significantly more patients achieving Dermatology Life Quality Index of 0 or 1 with guselkumab versus adalimumab at both Week 24 (61% and 40%, respectively; p<0.001) and Week 48 (63% and 39%, respectively; p<0.001) in the VOYAGE 1 study.24 At Week 52 in the VOYAGE 1 study, patients receiving adalimumab were switched to guselkumab; by Week 100, the proportion of patients achieving Dermatology Life Quality Index of 0 or 1 was similar in those switched from adalimumab to guselkumab (74%) compared with those who had received 2-years’ guselkumab (71%).29
With regard to the safety profile of guselkumab, a pooled analysis of the VOYAGE 1 and 2 studies, including 1,221 patients, indicated a low incidence of serious infections (1.06 infections per 100 patient years [including Week 0–100 data from patients randomised to guselkumab and those who crossed-over to receive guselkumab]).29 Similarly, the rates of malignancy and major adverse cardiovascular events were very low (both 0.38 events per 100 patient years).29
In summary, IL-23 inhibitors are an important component of the treatment repertoire for psoriasis. Such therapies demonstrate high levels of therapeutic efficacy, are well tolerated, and have durable responses that allow long injection intervals,24,25 which may have the potential to be extended further in the future.
Click here to view the full symposium.






