Abstract
The honeymoon phase, or partial clinical remission (PCR) phase, of Type 1 diabetes mellitus (T1DM) is a transitory period that is marked by endogenous insulin production by surviving β cells following a diabetes diagnosis and the introduction of insulin therapy. It is a critical window in the course of the disease that has short and long-term implications for the patient, such as a significant reduction in the risk of long-term complications of T1DM. To promote long-term cardiovascular health in children with newly diagnosed T1DM, three key steps are necessary: the generation of a predictive model for non-remission, the adoption of a user-friendly monitoring tool for remission and non-remission, and the establishment of the magnitude of the early-phase cardiovascular disease risk in these children in objective terms through changes in lipid profile. However, only about 50% of children diagnosed with T1DM experience the honeymoon phase. Accurate and prompt detection of the honeymoon phase has been hampered by the lack of an objective and easily applicable predictive model for its detection at the time of T1DM diagnosis, the complex formulas needed to confirm and monitor PCR, and the absence of a straightforward, user-friendly tool for monitoring PCR. This literature review discusses the most up-to-date information in this field by describing an objective predictive model for non-remission, an easy tool for monitoring remission or non-remission, and objective evidence for the cardiovascular protective effect of PCR in the early phase of the disease. The goal is to present non-remission as an independent clinical entity with significantly poorer long-term prognosis than partial remission.
INTRODUCTION
A literature search was conducted to identify publications addressing the honeymoon phase in children with Type 1 diabetes mellitus (T1DM). Medline, EMBASE, and Ovid were searched using the following search terms: ‘clinical remission’, ‘partial remission’, ‘partial clinical remission’, ‘honeymoon phase’, ‘C-peptide’, ‘type 1 diabetes’, ‘children’, ‘pediatric type 1 diabetes’, and ‘paediatrics type 1 diabetes’. Nine papers were excluded because they featured an unclear definition of partial clinical remission.
OVERVIEW AND DEFINITION OF PARTIAL CLINICAL REMISSION IN CHILDREN WITH TYPE 1 DIABETES MELLITUS
A key limitation of the early management of T1DM is the lack of a uniform strategy to prevent early dysglycaemia in non-remitters; these are children and adolescents who fail to experience the honeymoon phase or the partial clinical remission (PCR) phase of the disease.1-4 T1DM is a disorder of persistent hyperglycaemia resulting from autoimmune destruction of the pancreatic β cells.5,6 PCR often follows the diagnosis of T1DM and this phase is marked by an increased functionality of the surviving β cells with increased endogenous insulin production.4,7 PCR typically lasts 3–12 months;8 however, recent studies have shown evidence for C-peptide production and thus residual β cell function at >5 years following the diagnosis of T1DM.9 C-peptide is cosecreted with insulin from pancreatic β cells and represents a surrogate marker of residual β cell function. In physiologic concentrations, C-peptide acts to improve both microvascular blood flow and microvascular endothelial function through the release of endothelial nitric oxide.10 Following the diagnosis of T1DM, serum C-peptide concentration undergoes an initial exponential fall followed by a stable phase of decline that can last for several years.9 The presence of residual endogenous insulin secretion in patients with T1DM has been linked to a reduced risk of severe hypoglycaemia,11,12 development of diabetic retinopathy,13 increases in statural growth in prepubescent children,14 and improvement in long-term glycaemic control.2,15 These findings have been recently corroborated by a longitudinal study that reported a significantly reduced risk for chronic microvascular complications at 7-year follow-up in young adults who experienced PCR.2
Though remitters have an overall long-term prognostic advantage over non-remitters, this dichotomy is rarely taken into consideration during the early phase of diabetes management, as there is no specified strategy to prevent early dysglycaemia in non-remitters, which represents a key limitation of the early management T1DM in children.1-4 Research in this field has primarily focussed on prolonging the duration of PCR in remitters. A review of these studies showed differing conclusions because of the severe side effect profile of some of the agents,16-20 insufficient doses of other experimental agents,21,22 and, more importantly, the non-standardisation of insulin regimens16,17,21,22 during these interventions, which confounded the effect of the experimental agents. The immunomodulatory approach is promising, but the regimens tested lack sufficient benefit to justify the risk.16,17 Autologous haematopoietic stem cell transplantation,19 an approach that halts autoimmune destruction of targeted tissue and re-establishes tolerance, has huge potential in carefully selected patients, in whom it has resulted in remission for >3.5 years in the best cases. However, its effect is predicated on the existence of functional β cells and so would not be effective in non-remitters.23 More importantly, its numerous side effects, including alopecia, febrile neutropenia, nausea, de novo autoimmunity, infections, and death,20 have limited its widespread acceptance. Vitamin D supplementation, on the other hand, is safe and may slow T1DM progression,21,22 but existing studies have caveats that prevent widespread implementation of this recommendation. There is an ongoing randomised control trial evaluating the effect of moderately high-dose vitamin D supplementation on the duration of PCR; its results will be published in 2021.24 It is therefore crucial to understand the unique disadvantages of non-remitters to ensure that they are protected from early-phase metabolic derangements and the attendant long-term complications of T1DM.
Prevalence of Non-Remission in Children with Type 1 Diabetes Mellitus
The introduction of the gold-standard definition for PCR, the insulin dose-adjusted HbA1c (IDAA1c), in 2009 allowed for a consensus on the estimation of PCR.8 Based on recent studies, the prevalence of PCR in children and adolescents is approximately 50%.2,4,25,26 This means that a significant proportion of children and adolescents diagnosed with T1DM will not experience PCR.1,27-29
Mechanisms of Non-Remission
The molecular mechanisms underlying remission or non-remission are not fully characterised; however, certain factors play key roles, including increased β cell strain,30 unfavourable cytokine profile,31 increased glucagon concentration,32 and the role of immune mediators and genetic markers. Increased β cell strain is marked by poor processing of proinsulin because it has been shown that overweight male children who are more likely to undergo remission have improved processing of proinsulin.30 In addition, remitters are reported to possess a distinctive cytokine profile with a less damaging effect on the β cells.31 Remitters also have a lower glucagon concentration, a finding that is consistent with the premise that glucagon production is suppressed by intra-islet insulin production and release.32 A study suggesting a key role of immune mechanisms in PCR33 reported significantly lower concentrations of IFN-γ in remitters compared to non-remitters and controls. A higher frequency of CD4+ CD25+ CD127hi cells, a non-regulatory T cell subset of memory T cells, correlated with a slower rate of T1DM progression,34,35 supporting the hypothesis of a protective role for immune mediators for PCR. Moya et al.35 reported that a combination of the frequency of the CD4+ CD25+ CD127hi cells with glycaemic markers at the time of diagnosis of T1DM could serve as a predictor of the duration of PCR. A similar study reported that the highest levels of apoptosis of CD4+ CD25+hi T cells are seen in subjects with either new-onset T1DM or those with an increased number of diabetes-associated autoantibodies.36 Another study found that an increase in islet antigen-specific IL-10-producing cells in subjects with new-onset T1DM correlated with improved glycaemic control, while increased FoxP3 expression in similar subjects predicted a worse outcome.37 A genetic study38 reported that the level of circulating microRNA, has-miR-197-3p, at 3 months following the diagnosis of T1DM was a strong predictor of residual β cell function 1 year after the diagnosis of the disease. Thus, a constellation of genetic, immune, hormonal, and inflammatory factors predicts the occurrence and the duration of remission or non-remission.
The Shortcomings of the Singular Focus on the Determinants of Remission at the Expense of Non-Remission
The decision to focus this review on the determinants of clinical non-remission was predicated on the urgent need for a paradigm shift in the approach to the management of children with new-onset T1DM. A clear characterisation of non-remission as an entity would enable clinicians to institute measures to ensure optimal glycaemic control very early in the course of the disease in non-remitters,3 thus preventing early dysglycaemia. This new approach, which is based on the predictive model for non-remission, will have a significant impact on diabetes complications given the high prevalence of non-remission (>50%) in both paediatric and adult patients.1,27,29,39 A predictive model would also enhance candidate selection for β cell preservation trials, as well as in trials focussed on the prodromal phase of T1DM, such as the Type 1 Diabetes TrialNet Study.40
A recent study of 204 children and adolescents <18 years of age with new-onset T1DM examined the prevalence and key indicators of non-remission and reported a prevalence rate of non-remission of 57.8%.41 This study further demonstrated that the principal predictors of non-remission in this population were serum bicarbonate <15 mg/dL, younger age at diagnosis, increasing number of diabetes-associated autoantibodies, and female sex.41 In contrast, male sex and older age were associated with decreased risk of non-remission. One study reported that remission occurred more frequently in younger patients;42 however, other published studies reported otherwise.4,8,25,41,43-45 Serum 25-hydroxyvitamin D did not affect the risk of non-remission. This study further quantified the glycaemic cost of non-remission by demonstrating a prolonged period of a significantly elevated HbA1c level of 3–18 months post-diagnosis in non-remitters compared to remitters25 (Figure 1).
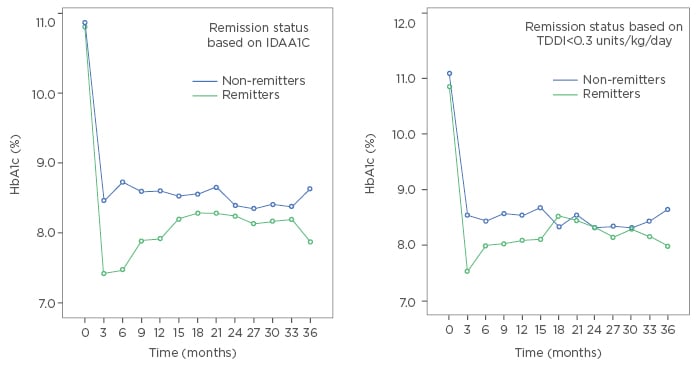
Figure 1: A longitudinal representation of the patterns of haemoglobin A1c (HbA1c) excursions in remitters and non-remitters in the first 3 years of diagnosis of Type 1 diabetes mellitus using either insulin dose-adjusted HbA1c ≤9 or total daily dose of insulin <0.3 units/kg/day.7,8
IDAA1c: insulin dose-adjusted HbA1c; TDDI: total daily dose of insulin.
MONITORING OF REMISSION AND NON-REMISSION
Despite the adoption of IDAA1c as the gold-standard marker for PCR, there is no consensus on a simple and user-friendly tool for the detection and monitoring of PCR in children and adolescents.4,26,41 This has limited the application of IDAA1c as a universal tool for the monitoring of PCR in children with new-onset T1DM. Therefore, the next challenge in preventing early-phase dysglycaemia in children with T1DM was to develop a simple, user-friendly tool for the monitoring of both remission or non-remission to ensure the institution and maintenance of intensive glycaemic control in these patients, especially the non-remitters who lack the honeymoon-associated endogenous protection from early dysglycaemia and the attendant long-term complications.1,11-13,27-29,46
The Drawbacks of the Gold Standard Marker for the Detection of Partial Clinical Remission: Insulin Dose-Adjusted HbA1c
The IDAA1c formula is expressed as HbA1c (%) + (4 x total daily dose of insulin [TDDI] [units/kg/24 hours]). This formula, which integrates both HbA1c and TDDI, has been validated in multiple cohort studies;4,7 however, this surrogate marker of serum C-peptide concentration has been criticised for its numerous shortcomings.4 The first major drawback is that age, which is a principal determinant of PCR, is not included in the formula.8 Secondly, IDAA1c underestimates PCR in younger children who often have lower serum C-peptide concentrations given their smaller pancreatic β cell mass, as the formula for IDAA1c was derived using a higher C-peptide cut-off value of 300 pmol/L, instead of the 200 pmol/L validated by the Diabetes Control and Complications Trial.3,8 Thirdly, IDAA1c underestimates PCR frequency in older, overweight European females with insulin resistance,4 as IDAA1c is unable to discriminate between insulin sensitivity and insulin secretion.4 IDAA1c also has poor sensitivity for the risk of hypoglycaemia in patients with newly diagnosed T1DM3 and the multistep approach to the calculation of IDAA1c represents a major barrier to its adoption by busy clinicians, which limits its widespread use in endocrine clinics. The final shortcoming of this formula is that IDAA1c may not be generalised to all children with T1DM because it was derived from a cohort of European and Japanese subjects, who have markedly different diabetes characteristics from the general population of the USA.3,47 Poor generalisation of the IDAA1c was recently demonstrated in the USA in a retrospective study that used IDAA1c to compare the glycaemic and cardiovascular parameters of 80 African-American, 216 Hispanic, and 631 non-Hispanic white (NHW) youths <19 years old with T1DM.48 The authors reported that African-American and Hispanic youths had higher mean HbA1c and BMI, and a lower frequency of remission, compared to NHW youths.48 Surprisingly, this study found no statistically or clinically significant differences in lipid parameters between the groups in the first 3 years of disease, despite the persistently higher HbA1c in the ethnic minority youths, suggesting that IDDA1c underestimated PCR in these youths in the USA, similar to the underestimation of PCR in obese European females.4 In other words, most of the African-American and Hispanic youths classified as non-remitters were false-negatives.47 This underestimation of PCR is exacerbated by the fact that USA ethnic minority youths with T1DM have a mean HbA1c of 0.4% higher than NHW youths for the same mean glucose concentration.49,50 They also have a more rapid rise in HbA1c trajectory from the time of diagnosis onwards.51 As a result of these shortcomings, there is now a call to design an ethnic group-specific IDAA1c value for ethnic minorities in the USA,47 and for additional research to clarify the usefulness and performance of IDAA1c in routine clinical practice.4 Specifically, there is a need to determine age, sex, and demographic-specific IDAA1c limits for the definition of PCR based on the serial determination of serum C-peptide concentration to ensure that the false-negative rate from the current criterion is reduced and that all remitters are accurately identified.4,47,52,53
The Comparison of Insulin Dose-Adjusted HbA1c to Other Markers of Partial Clinical Remission
A comparison of the IDAA1c to earlier definitions of PCR,26-30,47,54,55 such as HbA1c ≤7.5%, TDDI ≤0.5 units/kg/day,54 or combinations of the parameters, such as HbA1c ≤7.5% and TDDI ≤0.5 units/kg/day,55 showed that while IDAA1c has a stronger correlation with stimulated C-peptide concentration than previous definitions,8 IDAA1c is less sensitive than TDDI <0.5 units/kg/day for early detection of PCR, but is more specific for the detection of PCR between 6 and 12 months.8 Furthermore, the use of either TDDI ≤0.5 units/kg/day or HbA1c <7.5% in isolation was found to overestimate the prevalence of PCR, although the use of a combination of TDDI ≤0.5 units/kg/day and HbA1c <7.5% underestimated PCR.8 Recently, a Belgian group, citing the above shortcomings of the IDAA1c, developed a new tool for the detection of PCR called the glycaemic target-adjusted HbA1c,26 which has a sensitivity of 72% to detect PCR based on the IDAA1c definition.
Given the drawbacks of the IDAA1c and the shortcomings of the other definitions, a recent study compared IDAA1c to a new, straightforward, user-friendly definition of PCR: TDDI <0.3 units/kg/day.25,56 This comparison was based on the hypothesis that a TDDI <0.3 units/kg/day could fall in an intermediate position between the detection potential of TDDI ≤0.5 units/kg/day54 and the combination of HbA1c <7.5% and TDDI ≤0.5 units/kg/day.55 The rationale for this investigation was that endocrinologists, who routinely calculate TDDI during regular clinic visits, would find the application of TDDI <0.3 units/kg/day more practical and user-friendly for early detection and monitoring of either remission or non-remission than the IDAA1c, which would ensure a greater acceptance by clinicians. The results of this study, which was conducted in a USA population, showed that TDDI <0.3 units/kg/day and IDAA1c ≤9 identified a similar proportion of patients entering PCR: 40.2% versus 42.2%, respectively, with both criteria showing peak prevalence of remission at 6–12 months and a similar longitudinal HbA1c pattern in the first 3 years of disease (Figure 1). Specifically, Figure 1 shows that when PCR was defined by IDAA1c ≤9 criterion, mean HbA1c was similar at diagnosis between the remitters and non-remitters; thereafter, HbA1c became significantly lower in the remitters from 3–18 months and remained non-significantly lower in the remitters thereafter. When PCR was defined by TDDI <0.3 units/kg/day, HbA1c level appeared significantly lower in the remitters at diagnosis and at 3 months, and then became non-significantly lower in the remitters until Month 15, when the mean HbA1c values became similar between the groups. This study concluded that, in a head-to-head comparison, the criterion TDDI <0.3 units/kg/day was noninferior to IDAA1c, as both criteria showed similar sensitivity and specificity for the detection of PCR in children. However, TDDI <0.3 units/kg/day had the advantage of being a readily accessible tool for prompt detection and monitoring of PCR in a busy clinic setting. Therefore, the adoption of the routine application of TDDI <0.3 units/kg/day in clinical practice should improve the surveillance for both remission and non-remission to ensure the maintenance of euglycaemia in children requiring insulin doses >0.3 units/kg/day.25
CLINICAL CONSEQUENCE OF NON-REMISSION
The next challenge in the characterisation of PCR in children and adolescents is to provide objective proof of the deleterious cardiovascular effects of non-remission in the early phase of T1DM, specifically within the first 5 years. Such a demonstration could provide the basis for recommendations for early detection and monitoring of PCR in children to prevent the long-term microvascular and macrovascular complications that begin in the first few years of disease.57 This is particularly important because early changes in lipid profile between remitters and non-remitters have not been previously reported.52
Pathobiology of Early-Phase Dyslipidaemia in Children with Type 1 Diabetes Mellitus: The Need to Stratify Patients by Partial Clinical Remission History in the Investigation of Differences in Lipid Profile
Despite the report by the Diabetes Control and Complications Trial of a protective role of C-peptide on vasculature in remitters,15 there have been no data on the characterisation of early-phase dyslipidaemia in remitters and non-remitters until recently.52 A review of the current literature on dyslipidaemia in children and adolescents with T1DM showed no consensus on lipid pattern and it is believed that a lack of stratification of subjects by PCR history may have confounded these results.58-61 While one longitudinal study reported that 25% of youths with T1DM have progressive and persistent dyslipidaemia and increased arterial stiffness,58 another study in children and adolescents with poorly controlled T1DM found a positive association between increased arterial stiffness and total cholesterol, low-density lipoprotein (LDL) cholesterol, and HbA1c.59 In contrast, a longitudinal retrospective cohort study in a similar population with T1DM found that changes in HbA1c and BMI z scores had minimal impact on LDL cholesterol and non-high-density lipoprotein cholesterol.60 Furthermore, whereas some studies reported a significant relationship between poor glycaemic control and dyslipidaemia in T1DM,58,60 others reported an inconsistent pattern of correlation of lipids and HbA1c,62 or no correlation at all.63
However, none of the aforementioned studies explored the differences in lipid profiles based on patients’ remission statuses, except Redondo et al.,48 whose findings were confounded by the underestimation of PCR by IDAA1c in ethnic minority youths.47 The stratification of children and adolescents with new-onset T1DM by PCR status is of fundamental importance to ensure meaningful comparisons between the groups to reduce the inconsistencies in lipid outcomes, especially LDL cholesterol concentration, in previous studies.58-61 For instance, it is possible that the study that reported progressive and persistent dyslipidaemia58 could have contained a higher proportion of non-remitters, while the study that reported only a modest effect of HbA1c and BMI on lipid parameters60 might have had a higher proportion of remitters. The fact that non-remitters make up >50% of children and adolescents with new-onset T1DM27,29 makes it crucial to stratify subjects based on PCR history in all investigations in circulating lipid concentrations in patients with T1DM.
Furthermore, the characterisation of early-phase dyslipidaemia in T1DM, specifically the changes in LDL cholesterol, based on stratification by PCR is imperative because atherosclerosis originates in childhood and early adolescence,64,65 and dyslipidaemia is a primary contributor to the increased risk of cardiovascular disease in patients with T1DM.64,65 Such an emphasis may clarify the possible role of non-remission as a non-modifiable risk factor for dyslipidaemia in T1DM.
The first step to address this came from a recent longitudinal retrospective cohort study of 123 children and adolescents with T1DM of 5-year duration.66 The subjects’ mean age was 11.9±2.9 years and the cohort consisted of 55 male subjects and 68 female subjects. There were 44 remitters and 79 non-remitters. A timeline of 4–5 years after diagnosis was chosen in concert with the American Diabetes Association (ADA) recommendation to initiate screening for diabetes complications in children either at the inception of puberty or 4–5 years after diagnosis,67 because it was previously believed that there was minimal risk of dyslipidaemia during the prepubescent years. This study excluded children with dyslipidaemia or a family history of lipid abnormalities. The results showed that children and adolescents who experienced PCR had significantly lower mean LDL cholesterol 4–5 years after the diagnosis of T1DM compared to their peers who did not experience PCR52 after controlling for age, puberty, glycaemic control, and adiposity. The significantly lower LDL in remitters was rather striking because a greater proportion of the remitters were in puberty 4–5 years after the diagnosis of T1DM compared to the non-remitters. This is because a previous report indicated that pubescent youths with T1DM had elevated LDL cholesterol compared to their healthy peers,63 and this was attributed to the fact that children with T1DM do not show the usual pattern of decreasing LDL cholesterol during puberty.63 Hence, this study clarified the previous report by demonstrating that remitters in puberty had similar mean LDL cholesterol concentrations as children and adolescents without diabetes, a finding that has not been previously reported. This report advances the field by providing critical and objective evidence of early cardiovascular protection by PCR. Larger studies are needed to confirm these differences in lipid fractions between remitters and non-remitters.
CONCLUSIONS AND SUGGESTED CHANGES TO THE LIPID MONITORING GUIDELINES OF THE INTERNATIONAL SOCIETY FOR PEDIATRIC AND ADOLESCENT DIABETES (ISPAD) AND THE AMERICAN DIABETES ASSOCIATION (ADA)
This literature review details new developments in the clinical application of the honeymoon period of T1DM by focussing on non-remission as a specific clinical entity with poorer long-term prognosis than partial remission. The author hopes to advance the field by presenting strategies to predict and monitor non-remission, as well as a justification for the initiation of lipid monitoring at the time of diagnosis of T1DM in children and adolescents. This review has summarised the genetic, immune, inflammatory, and biochemical markers that could predict remission and non-remission. It has also characterised the first clinical predictive model dedicated to non-remission in children with new-onset T1DM that is based on easily obtainable clinical parameters at the time of T1DM diagnosis, such as age, sex, BMI, body pH, number of diabetes-associated autoantibodies, and serum bicarbonate. It further reviewed a study showing that a TDDI <0.3 units/kg/day is noninferior to the gold standard marker of PCR, IDAA1c ≤9. Finally, it reviewed the results of a 5-year longitudinal study showing evidence for early-phase dyslipidaemia in non-remitters who had a significantly elevated LDL cholesterol level compared to remitters after adjusting for confounding variables. Though larger studies are needed to confirm these early findings, the distinct pattern of the early changes in LDL cholesterol in this recent study52 could explain the dichotomy in the prevalence of long-term complications of T1DM between remitters and non-remitters.15 Significantly elevated LDL cholesterol in non-remitters makes a strong case for targeted lipid monitoring in T1DM because it suggests that non-remission could be a non-modifiable risk factor for cardiovascular disease in T1DM. Equally, the early divergence in serum LDL cholesterol concentration in these paediatric subjects with T1DM supports a modification of the current guidelines by the International Society for Pediatric and Adolescent Diabetes (ISPAD)68 and the ADA67 to recommend the initiation of lipid monitoring at the time of diagnosis of T1DM regardless of the age of the child, in a similar approach to the current guidelines for Type 2 diabetes mellitus.68 This will ensure an early detection of hypercholesterolaemia in non-remitters that could be amenable to the institution of early therapeutic and dietary interventions coupled with ongoing lipid monitoring.
In conclusion, the adoption of the following measures may serve to improve the long-term cardiovascular health of diabetic children and adolescents well into adulthood: the implementation of a predictive model to detect non-remission; using TDDI <0.3 units/kg/day to more easily monitor remission or non-remission; and the introduction of targeted lipid monitoring at the time of diagnosis in all children with newly diagnosed T1DM. Larger studies including patients of different nationalities are needed to confirm the generalisability of these measures.







