Meeting Summary
The FutureIBD meeting in Barcelona, Spain, was the first time that leading gastroenterologists had been able to meet face-to-face for quite some time due to the challenges of the COVID-19 pandemic. As a result of this, the atmosphere at the meeting was understandably buoyant. There was a great deal of discussion and debate among presenters and attendees, making the meeting feel very inclusive and interactive.
Workshops and activities had been arranged, creating a well-rounded experience. Some of the topics discussed included raising standards of care, striving for better outcomes, future goals and monitoring, and immunological pathway developments. There was a focus on how best to help patients, with individualised monitoring strategies and clear treatment targets. Speakers also discussed the impact of clinical trials on clinical practice and, conversely, how the aspiration to advance clinical practice drives the stringency of endpoints defined in clinical trials.
Overall, the meeting underlined mucosal healing as a long-term treatment target in both Crohn’s disease (CD) and ulcerative colitis (UC), with the understanding that consistently reducing inflammation may give the gastrointestinal system a chance to heal.
Faculty: Tim Raine,1 Peter Bossuyt,2 Krisztina Gecse,3 Reena Khanna,4 Joana Torres,5 Ryan Ungaro,6 Raja Atreya,7 James Lindsay,8 Remo Panaccione,9 Jean-Frederick Colombel,6 Ignacio Marín-Jiménez,10 Subrata Ghosh11
1. Cambridge University Hospitals NHS Foundation Trust, UK
2. Imelda General Hospital, Bonheiden, Belgium
3. Amsterdam University Medical Centre, The Netherlands
4. University of Western Ontario, London, Canada
5. Hospital Beatriz Ângelo, Loures, Portugal
6. Icahn School of Medicine at Mount Sinai, New York City, New York, USA
7. University Hospital Erlangen, Germany
8. Barts and the London School of Medicine and Dentistry, UK
9. University of Calgary, Canada
10. Hospital General Universitario Gregorio Marañon, Madrid, Spain
11. Chair of Medicine, College of Medicine and Health Institution, University College Cork, Ireland
Disclosure: Raine has received research or educational grants and/or speaker or consulting fees from AbbVie, Arena Pharmaceuticals, ASLAN Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Heptares Therapeutics, Janssen, LabGenius, MSD, Mylan, Novartis, Pfizer, Sandoz, Takeda, and UCB. Bossuyt has received research support, lecture fees, and/or advisory board fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Celltrion, Dr Falk Pharma, Galapagos, Janssen, Lilly, Mylan, Pentax, Pfizer, PSI CRO, Roche, Takeda, and Tetrameros. Gecse has received grants, consulting fees, and/or speaker honoraria from AbbVie, Arena Pharmaceuticals, Galapagos, Pfizer, Celltrion, Gilead, Immunic Therapeutics, Janssen, Novartis, Samsung Bioepis, Takeda, Ferring, and Tillotts. Khanna has received consulting or speaker fees and/or research fees from AbbVie, Alimentiv (formerly Robarts), Amgen, Encycle, Gilead, Innomar, Janssen, Lilly, Merck, Pendopharm, Pfizer, Roche/Genentech, Shire, and Takeda Canada. Torres has received grants and speaker fees from and/or has served on advisory boards of AbbVie, Janssen, Arena Pharmaceuticals, Galapagos, and Pfizer. Ungaro has received research support from and/or served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Janssen, Pfizer, Takeda, Boehringer Ingelheim, and Eli Lilly and Company. Atreya has received grants and/or and consulting or speaker fees from Biogen, InDex Pharmaceuticals, Takeda, Tillotts, AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Ferring, Fresenius Kabi, Galapagos, Gilead, GlaxoSmithKline, Janssen-Cilag, Kiniksa Pharmaceuticals, MSD, Novartis, Pandion Therapeutics, Pfizer, Roche, Stelic Institute, and Viatris. Lindsay has received honoraria, research support, consultancy and/or speaker fees, and travel support from Cornerstones Health, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Galapagos, Gilead, Janssen, Pfizer, Takeda, Tillotts, Shire, Allergan (Warner Chilcott), Atlantic Healthcare, Celltrion, Ferring, GlaxoSmithKline, Lilly, MSD, Napp, Norgine, and Vifor Pharma. Panaccione has received grants, speaker fees, consulting fees, and/or research support from and/or served as an advisory board member for AbbVie, Abbott, Alimentiv, Amgen, Arena Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly and Company, Ferring, Galapagos, Genentech, Gilead, GlaxoSmithKline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Therapeutics, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Schering-Plough, Shire, Sublimity, Takeda, Theravance Biopharma, and UCB. Colombel has received research grants, lecture fees, and/or consulting fees from AbbVie, Allergan, Amgen, Ferring Pharmaceuticals, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Ferring, Galmed Pharmaceuticals, Geneva, GlaxoSmithKline, Iterative Scopes, Janssen, Kaleido Biosciences, Landos Biopharma, Otsuka Pharmaceuticals, Pfizer, Prometheus Biosciences, Sanofi, Shire, Takeda, and TiGenix; and has held stock options in Intestinal Biotech Development. Marín- Jiménez has received speaker fees, consulting fees, and research funding from and/ or has served as an advisory member for AbbVie, Amgen, Chiesi, Dr Falk Pharma, Faes Farma, Ferring, Gebro Pharma, Hospira, Janssen, Kern Pharma, MSD, Otsuka Pharmaceuticals, Sandoz, Shire, Takeda, and Tillotts. Ghosh has received speaker fees from Takeda, Abbvie, Janssen, Ferring, Gilead, Celltrion, Pfizer, and Galapagos; served on advisory committees for Janssen, Abbvie, Takeda, Pfizer, Eli Lilly, Celltrion, Ferring, Arena, Galapagos, and Gilead; and served on steering committees for Janssen, Abbvie, Galapagos, and Eli Lilly.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK
Support: The FutureIBD meeting and the publication of this article were funded by AbbVie. The views and opinions expressed are exclusively those of the speakers. The content was reviewed by AbbVie for medical accuracy.
Citation: EMJ Gastroenterol. 2022;11[Suppl 2]:2-12.
Focus for Standard of Care Today and in the Future
Tim Raine
Tim Raine, Cambridge University Hospitals NHS Foundation Trust, UK, started by emphasising that the care gastroenterologists should be delivering to their patients tomorrow cannot, and should not, look the same as the care they have delivered up until now. The ultimate goal is to improve outcomes for every patient diagnosed with inflammatory bowel disease (IBD), so that they can achieve a normal quality of life for the rest of their natural life span.
To achieve this, gastroenterologists spend a lot of time thinking about new drug targets and treatment strategies. However, there are many other aspects of care that also need to improve, which are summarised in Figure 1.
To redefine expectations in IBD, goals need to be raised at each stage of the disease. Raine emphasised that in the earliest stages of IBD, the aim should be complete symptom control, including normalisation of C-reactive protein (CRP) and faecal calprotectin (FC). Intermediate goals need to be raised to deep remission, with endoscopic and mucosal healing; and for long-term goals, the aim should be normal quality of life, absence of disability, and freedom from disease complications.
Although attaining long-term goals should be the primary objective, Raine stressed that this focus should not be at the expense of short- and intermediate-term goals. He noted that a dynamic approach to improving care will enable a shift towards truly personalised medicine in IBD.
Raine described care delivery as a key area that needs to improve. The ‘5C’ concept in IBD care emphasises the importance of a multidisciplinary approach, incorporating comprehensive care at specialist centres, collaboration between specialists, communication through clinical nurse specialists, and patient care pathways.2 However, he explained that optimal care also requires consideration of patients’ perspectives, which do not always align with those of physicians. For example, endoscopic or histologic confirmation of disease remission is far less important to patients than it is to physicians.3 Raine stressed that before asking patients to undergo colonoscopy or further invasive procedures, gastroenterologists need to fully communicate the importance of these measures to them.
“The interaction among colleagues and faculty provided an engaging environment to share ideas and challenge one another to improve patient care.”
Due to the dramatic increase in telemedicine necessitated by the COVID-19 pandemic, gastroenterologists have seen less of their patients face-to-face. Although some gastroenterologists may have been uncomfortable with this change, it has provided a perfect opportunity to consider how IBD services can be redesigned in order to achieve optimum outcomes. For example, centres that deliver remote monitoring can, and do, achieve good outcomes,4-7 though physicians’ ability to implement these practices will differ according to local infrastructure and support.
One area in which IBD care services can be improved is by reducing the overuse of corticosteroids, and this has been a particular focus in the UK in recent years. Corticosteroids are regularly used in IBD; a large study of patients in the USA with CD showed that 42% initiated treatment with corticosteroids.8 However, Raine described the use of corticosteroids in IBD as pouring water on a fire: usually the fire will go out, but if it keeps rekindling then another means to extinguish it should be sought. In 2017, 15% of IBD outpatients in the UK had corticosteroid dependency or excess, and this was considered avoidable in about half of cases.9
Part of the issue is that gastroenterologists may not fully appreciate how many corticosteroids they are actually prescribing; patients report a significantly higher degree of steroid use than their clinicians do.10 Addressing this issue through the use of multidisciplinary teams and/or quality improvement programmes has been shown to reduce the risk of corticosteroid overuse,9 highlighting the importance of how IBD care services are configured and delivered. Simply by monitoring corticosteroid use in outpatient services, quality of care can be improved11 and, for this reason, IBD guidelines in the UK now recommend regularly auditing corticosteroid use.12
Raine concluded by emphasising that there are real grounds for optimism in terms of advancing the standard of care in IBD. By redefining expectations for patients, outcomes can be improved and, by employing corticosteroid usage as a surrogate marker for quality of care, patients with suboptimal outcomes can be identified.
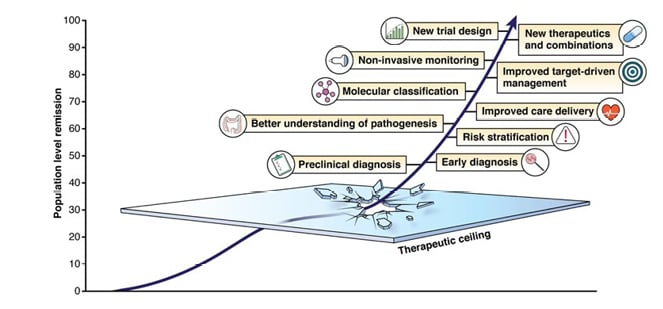
Figure 1: Focus points for advancing care in IBD.
Reproduced with permission from Raine and Danese.1
STRIDE-II and Beyond: Translating Consensus Recommendations into Clinical Practice
Peter Bossuyt and Tim Raine
Gastroenterologists will all be familiar with the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE-II) algorithm for IBD treatment, which were founded on evidence-based consensus recommendation (Figure 2).13 This represents an idealised model, and Raine and Peter Bossuyt, Imelda General Hospital, Bonheiden, Belgium, discussed how to adapt the practical guidance from STRIDE-II to the real world, where patients’ progress may not go to plan.
How Can Gastroenterologists Help Patients to Progress from Symptomatic Relief to Endoscopic Healing?
One of the challenges of IBD treatment is managing the period between symptomatic relief and the ultimate target: endoscopic healing. Bossuyt explained that this is one of the key differences between STRIDE-I, which consisted of static targets, and STRIDE-II, which provides more granularity in patient evaluations. He stressed that it is important to discuss a monitoring strategy with the patient at an early stage to ensure they understand that monitoring is vital to the progression from symptomatic relief to endoscopic healing.
“Real world patient cases are always insightful to learn how others might consider treatment options.”
How Can STRIDE-II Approaches Be Implemented When Access to Tools and Patient Engagement Vary?
The biomarkers a clinician uses to monitor IBD will depend both on the patient preference and the clinician’s resources. FC measurement, for example, is not always available and some patients prefer not to provide stool samples. In these situations, Raine suggested that STRIDE-II should be used more as a route-map rather than an exact prescription of IBD management; for example, gastroenterologists can elect to measure CRP or erythrocyte sedimentation rate instead of FC.
What Are the Different Considerations for Crohn’s Disease and Ulcerative Colitis When Implementing a Treat-to-Target Approach?
Implementation of a treat-to-target approach depends on the type and subtype of IBD, as well as the severity of the presenting phenotype, as this can determine how aggressively the disease needs to be treated.13 Endoscopic assessment, for example, is performed at a much earlier stage in UC than in CD. Even in a patient with minimal UC symptoms, Bossuyt explained that he would still perform an endoscopy as an endoscopic histology acts as the best predictor of disease course, and findings can affect decisions regarding further treatment.
Setting Expectations for Treatment Targets in Different Patient Groups
The principles of STRIDE-II consensus strategy can be adapted to apply to individual patient groups. Raine used the example of perianal CD, where the primary goal remains to make the patient feel better. Hence, surgical intervention might be needed if the patient is too uncomfortable to sit down. Bossuyt highlighted that patients with UC or primary sclerosing cholangitis have a higher risk of colon cancer than other IBD types and, therefore, the expectations of treatment success need to be higher in these groups.
Raine stressed that patients who are elderly are particularly at risk of corticosteroid overuse and may be less tolerant of endoscopy due to frailty. The risks associated with treatment may differ in this patient group, including opportunistic infections, but it is important not to undertreat patients just because of their older age.
Optimising Management in a Patient Who Has Achieved Some Targets but Not Others
Another challenge of IBD lies in when to optimise the current treatment and when to change the approach. In cases where a patient is still symptomatic, even after achieving mucosal healing, Bossuyt suggested switching treatment, if other options are available, and optimising the current treatment if there are not. However, he stressed that it is always important to consider patient preferences before changing treatment.
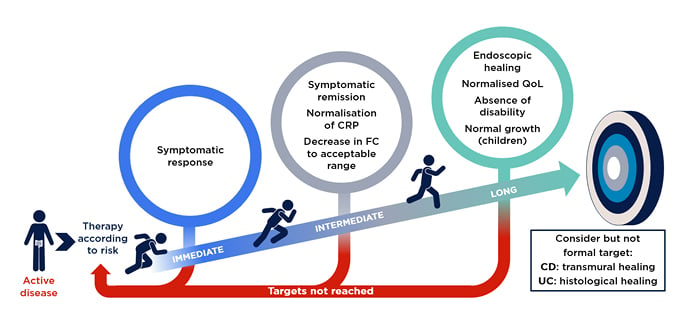
Figure 2: STRIDE-II evidence-based consensus recommendation.
Reproduced with permission from AbbVie.
CD: Crohn’s disease; CRP: C-reactive protein; FC: faecal calprotectin; QoL: quality of life; STRIDE-II: Selecting Therapeutic Targets in Inflammatory Bowel Disease algorithm; UC: ulcerative colitis.
Demystifying Endpoints, Goals, and Targets: From Clinical Trials to Clinical Practice
Krisztina Gecse and Reena Khanna
Reena Khanna, University of Western Ontario, London, Canada, began her presentation by defining the terms ‘endpoints’, ‘goals’, and ‘targets’, which are sometimes used interchangeably in the literature. She explained that ‘endpoint’ is a term reserved for clinical trials; it is a measure of success at a predefined timepoint, comparing a placebo with an active drug. Endpoints do not necessarily impact the course of the disease. On the other hand, a ‘goal’ is a long-term ambition that can be identified by a patient or clinician, while a ‘target’ is a short-term, achievable ambition that impacts clinical outcomes. Targets need to be adjustable through therapeutic optimisation.14
“The practicality of these sessions enabled me to incorporate these learnings into my clinical practice.”
There is considerable interplay between each of these terms, and Khanna explained that trial endpoints can often provide hints towards future treatment targets that may be used in the clinic. Conversely, the experience of clinical practice can sometimes drive the choice of endpoints in clinical trials.13,15
Evolution in Clinical Trial Design and Endpoints
In close collaboration with regulatory agencies regarding registrational trials for new therapies, clinical trial endpoints have changed over the years. The evolution from a focus on clinical outcomes measured by clinical disease activity indices (e.g., the Crohn’s Disease Activity Index [CDAI]) to a focus on patient-reported outcomes and endoscopic indices, which better reflect the ultimate goal of endoscopic remission.16-19
Clinical trial design has also evolved, from early trials looking at induction alone, to open-label induction followed by randomised maintenance, to re-randomisation between induction and maintenance phases. Khanna stressed that clinical trial design was likely to continue evolving.
Using Endpoints, Goals, and Targets in Clinical Practice
STRIDE-II confirmed endoscopic healing as a long-term clinical target, and now include clinical remission, normalisation of non-invasive biomarkers CRP and FC as intermediate goals, and restoration of quality of life and absence of disability as additional long-term goals (Figure 2).13 Krisztina Gecse, Amsterdam University Medical Centre, The Netherlands, highlighted that STRIDE-II also suggest that gastroenterologists should consider informal targets of transmural or histological healing in CD and UC, respectively.
The inclusion of normalised FC as an intermediate UC treatment target in clinical practice is supported by a prospective observational study, which found that baseline FC levels of >300 mg/kg in patients considered to be in clinical and endoscopic remission were associated with a significantly higher risk of relapse after 6 and 12 months.20 The use of biomarkers as treatment targets has also been shown to benefit outcomes in CD. The CALM study showed that a tight control regimen (based on biomarkers plus clinical symptoms) rather than clinical management alone was associated with better clinical and endoscopic outcomes at 48 weeks.21
The importance of mucosal healing as a goal for long-term outcomes in CD was demonstrated by a meta-analysis. Mucosal healing at first endoscopic assessment was associated with long-term clinical remission (≥50 weeks) and with CD related surgery-free rate (pooled odds ratios of 2.8 and 2.2, respectively).16 Similarly, a meta-analysis of prospective studies of patients with UC found that mucosal healing at first endoscopic assessment was associated with long-term remission (≥52 weeks) and remaining free of colectomy (pooled odds ratios of 4.5 and 4.15, respectively) in these patients.22
Gecse highlighted an IBD disk tool that was developed in alignment with the IBD Disability Index (IBD-DI) and has since been validated to help gastroenterologists monitor patients’ goals, which can change over time.23,24 This tool generates a visual indicator of IBD symptoms (e.g., abdominal pain, quality of sleep, impact on education and work produced, etc.) from patients’ responses to a series of 10 questions.
It can be challenging to use comprehensive disease scoring systems to measure disease activity in the clinic because these are a subjective assessment, and Khanna explained that gastroenterologists often do not agree on how to score lesions. On the other hand, endoscopic indices such as the Crohn’s Disease Index of Severity (CDEIS) and the Simple Endoscopic Score for Crohn’s Disease (SES-CD) have been shown to have excellent intra- and inter-rater reliability.25
While Khanna recommended that endoscopic scoring indices should be used more often in clinical practice, she emphasised the importance of understanding their limitations. For example, some measurements such as scarring or pseudo-polyps are less relevant to clinical practice than to clinical trials, as they are unlikely to resolve with anti-inflammatory therapy. It is also important to remain consistent; for example, switching between CDEIS and SES-CD at different stages of a patient’s disease considerably reduces the reliability of endoscopic scoring. Finally, Khanna emphasised that the description of endoscopic findings is equally important as the use of scoring indices in clinical practice.
In summary, histology and transmural healing are currently very important endpoints in clinical trials, but gastroenterologists are still working on how to best use these measures in clinical practice.
State-of-the-Art Techniques for Non-invasive Monitoring in Inflammatory Bowel Disease
Joana Torres and Ryan Ungaro
Ryan Ungaro, Icahn School of Medicine at Mount Sinai, New York City, New York, USA, explained that, although the introduction of treat-to-target and tight control regimens in IBD has improved patient outcomes, the increased disease monitoring associated with these methods can be a burden on patients.
The invasive assessment of endoscopic healing remains the gold standard for measuring remission in IBD. Non-invasive markers such as CRP and FC are currently assessed at the same time as an endoscopy so that findings can be correlated.26 However, the field of non-invasive monitoring is advancing (Figure 3).
Biomarkers Can Indicate Disease Activity and Predict Response to Medication
Studies have shown that biomarkers such as CRP, FC, and, to a lesser degree, stool lactoferrin are valuable indicators of disease activity in IBD, with FC generally outperforming CRP, particularly in UC.27
In everyday clinical practice, Ungaro explained that high FC levels could be used to predict response to medication. In a study in patients with CD (n=116), FC levels at 8 weeks after infliximab dose escalation were significantly lower in patients who achieved endoscopic response, endoscopic remission, or absence of ulceration at Week 54 (all p<0.01).28 Similarly, a study in patients with UC (n=87) found that two consecutive FC measurements of >300 mg/kg, 1 month apart, could predict relapse with 61.5% sensitivity and 100.0% specificity within the first year of infliximab treatment.29 Lastly, a recent study has shown that FC may have prognostic capability; patients with CD (n=375) that achieved normalised FC levels had a significantly lower risk of composite disease progression (p<0.001).30
Intestinal Ultrasound Can Accurately Measure Disease Activity and Can Be Used at Point-of-Care
Joana Torres, Hospital Beatriz Ângelo, Loures, Portugal, explained that intestinal ultrasounds (IUS) have been shown to be accurate in measuring disease activity and extent (e.g., comparable to ileo-colonoscopy), and to have a good correlation with endoscopic healing. One of the advantages of IUS is that patients with IBD rate it highly for acceptability and perceived clinical utility.31 Importantly, IUS is inexpensive compared with endoscopy or MRI. It is also widely available and can be used at point-of-care.
Several features of IBD can be assessed with IUS, including bowel wall thickness and disruption of bowel wall stratification (both of which correlate with disease activity); length of bowel affected by the disease; presence of increased vascularisation (indicating more severe disease); and extramural findings (e.g., lymph nodes and mesenteric fat hypertrophy). IUS assessment can also be used to monitor changes in disease activity in response to treatment in patients with CD,32,33 and potentially in patients with UC.34
There are several perceived barriers to the implementation of IUS.35 One of these is a concern that IUS has the potential for operator dependence, which Torres felt was a misconception. Another is a lack of training opportunities, although Torres believed that this had improved in recent years. There is currently no single accepted IUS score validated for IBD; however, Torres explained that this was perhaps more important for clinical trials than in clinical practice. Finally, more studies are needed to determine how best to use IUS alongside other imaging modalities in IBD.
Point-of-care IUS could be used to monitor IBD in a treat-to-target concept by scheduling assessments every 6–12 months in asymptomatic patients with negative biomarkers; as needed in patients with symptoms and/or positive biomarkers; and every 3 months in symptomatic patients initiating treatment.36
Ungaro predicted that the future of IBD care would include increasing use of both IUS and blood-based assessment of endoscopic healing in clinical practice, reducing the frequency of endoscopy. There is also likely to be a greater use of prognostic or predictive biomarkers, remote monitoring devices, telemedicine, and at-home treatment options. In addition, specific drugs will likely have early-stage tests to predict response.
To achieve these changes, longer-term follow-up of patients is needed to understand the impact of these methods on clinical outcomes. More comparative studies of monitoring tools and strategies and increased incorporation of biomarkers into both clinical trial and real-world cohort studies will also be important.
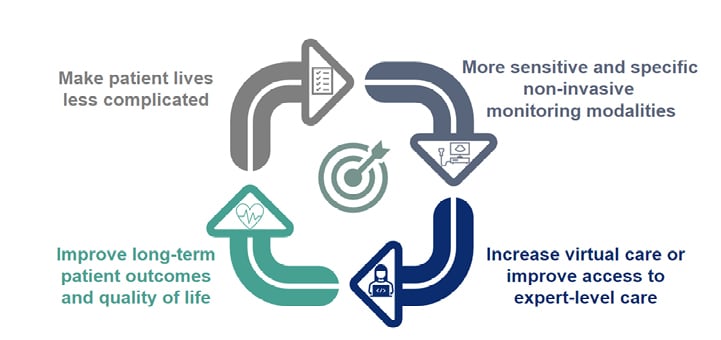
Figure 3: Ultimate goals for non-invasive monitoring.
Reproduced with permission from AbbVie.
Targeting New Immune Pathways in Inflammatory Bowel Disease and How This Will Impact Clinical Practice
Raja Atreya and James Lindsay
IBD is a complex disease that involves the activation of overlapping immunogenic and inflammatory pathways. A combination of genetic, environmental, and microbiome factors can lead to a breach in the epithelial barrier of the bowel, resulting in a heightened translocation of microbial products to the mucosa. This triggers an inflammatory reaction that can result in chronic inflammation of the mucosa.37,38
Raja Atreya, University Hospital Erlangen, Germany, explained that an awareness of new immune targets in IBD is important to gastroenterologists working in clinical practice because of the growing range of drugs available. An understanding of the mechanisms of action of these drugs, which impacts their safety and efficacy, can help to select the most suitable treatment for each patient.
Inhibition of IL-23 Has the Potential to Alleviate Mucosal Inflammation and Improve Epithelial Barrier Integrity
One promising immune target in IBD is IL-23. This cytokine is involved in inflammation in UC, CD, and psoriatic arthritis.39 IL-23 stimulates the differentiation of inducible Th17 cells into pathogenic Th17 cells, which produce high levels of the inflammatory cytokines IL-17 and IL-22, interferon-γ, and TNF.40
In preclinical models, inhibition of IL-23 ameliorates mucosal inflammation and improves epithelial barrier integrity.39 Inhibition of IL-23 does not result in downstream suppression of IL-17 because this cytokine can also be produced by non-pathogenic Th17 cells; this is important because IL-17 is involved in the preservation of epithelial barrier integrity.41 Studies have also indicated that inhibition of IL-23 leads to expansion of anti-inflammatory regulatory T cells,42 suggesting a potential value in the maintenance phase of IBD treatment.
Patients with IBD have elevated levels of IL-23 and Th17-induced cytokines in the intestinal mucosa and serum,40 indicative of inflammation. However, a significantly greater mucosal upregulation of IL-23 has been shown to occur in anti-TNF non-responders compared with responders,43 suggesting that IL-23 may also be involved in treatment resistance, perhaps through immune evasion.
Small Molecule Inhibitors of JAK Inhibition Show Promise for Inflammatory Bowel Disease Therapy
In addition to extracellular cytokine targets such as IL-23, James Lindsay, Barts and the London School of Medicine and Dentistry, UK, discussed how intracellular pathways involved in IBD associated inflammation can be modified. One target being investigated for IBD therapy is JAK. JAK proteins initiate signal transduction from extracellular cytokines to modulate gene transcription by interacting with signal transducers and activators of the transcription pathway (STATs).44 Specific cytokines utilise a specific combination of JAKs, impacting a diverse range of biological functions.44
Biologics such as TNF inhibitors bind extracellular targets. Their efficacy is related to exposure and function at the target site, and may not correlate, in a linear sense, with dose. Side effects are therefore related to the mechanism that the drug affects, rather than the dose; they rarely have off-target effects.44,45
On the other hand, small molecules like JAK inhibitors are suitable for binding to intracellular targets. These molecules can be engineered to be selective, but they are not as specific to their target as biologics are. Their efficacy depends on their relative inhibitory potential, meaning that higher doses have a greater impact and may have broader off-target effects. Therefore, side effects generally increase with the dose of a small molecule inhibitor.44,45
Conclusions
Across the FutureIBD meeting, there was a focus on improving the overall standard of care in IBD, by individualising goals, treatment, and monitoring strategies. Improvements should focus on using the principles of STRIDE-II consensus recommendation to adapt IBD management for each patient and each clinic, improving quality-of-care through regular auditing, and gradually increasing the use of non-invasive monitoring tools such as biomarkers or IUS for the future management of IBD.
The FutureIBD evaluation report can be found here
https://bit.ly/3FVpw2R








