Abstract
Epstein–Barr virus (EBV) is one of the most important viral causes for the development of tumours. The global geographical epidemiology of EBV shows prevalence differences between rich and poor countries across the world, and the impact on health suggests EBV should be an important target of research worldwide. This article will discuss the biology of the virus with an emphasis on its latency types, vital to understanding the possibilities of viral detection. The main objective is to discuss two lymphoproliferative diseases that are associated with EBV and appear in the World Health Organization (WHO) 2017 Classification of Tumours of Haematopoietic and Lymphoid Tissues: EBV-positive diffuse large B cell lymphoma and EBV mucocutaneous ulcer. The name of the former was changed to support the better understanding of infection pathology, while the second was recently described and made its debut in the WHO classification. Pathologists must have knowledge on these diseases and how to investigate them, and oncologists and clinical doctors must be informed on the guidelines.
INTRODUCTION
Viral aetiology for tumours was described for the first time in 1911.1 Almost 56 years later, a relationship between Epstein–Barr virus (EBV) and lymphoid neoplasms was reported by studies involving Burkitt’s lymphoma (BL).1 Nowadays, other viruses and their influences on the development of tumours are the focus of research; some examples of such viruses include hepatitis B virus, hepatitis C virus, herpes virus 8, human papilloma virus, and human T cell lymphotropic virus Type 1. In the context of lymphoproliferative disorders, EBV is a major contributor.1
In humans, EBV is the most common persistent virus infection, with approximately 95% of the world’s population presenting with an asymptomatic life-long carrier status.2 EBV has a geographical epidemiology of around 10–15% prevalence among individuals from Asia and South America, and a lower prevalence of 5% in the USA and Europe.3 Geographical differences are interesting in the study of EBV infection because there is an association with social and economic aspects. Countries from North Africa and China have higher endemic rates than countries from Northern Europe, such as Denmark and the Netherlands.4 One study of Hodgkin’s lymphoma (HL) reported EBV in 100% and 88% of mixed cellularity subtype patients from northeast Brazil and southeast Brazil, respectively.4 Brazil has a continental size and the regions are very different in relation to culture, social aspects, and economic levels. Despite recent advances, the inequality seen in Brazil is recognised as a global microcosm of the EBV infection status worldwide.
Malignancies associated with EBV represent approximately 200,000 new cases and 143,000 deaths from cancer worldwide every year.5 With regard to the high public health and economic impact, the National Institutes of Health (NIH) indicates EBV as an important target for cancer prevention, stimulating studies and research on the virus, predictive markers, a therapeutic vaccine, and immune correlations in EBV-related malignancies.5
Generally, the first contact with EBV occurs at a young age. The virus starts its replication cycle in the oropharynx, affecting epithelial cells and B cells of lymphoid tissues associated with the mucosa. EBV may produce three reactions: replication in permissible B cells or epithelial cells, latent infection in B cells, and stimulation or immortalisation of B cells.6,7
The scientific history of EBV began when virions were detected in B cells of BL biopsies. After many years, it was discovered that EBV is the main parasite of B lymphocytes and, in tissue culture models, the virus stimulates B cell growth and immortalisation.6,7 The definition of B lymphocyte tropism is supported by CD21, an EBV-specific receptor that also binds C3d. These receptors are found on epithelial cells from the oropharynx and nasopharynx of human and monkey B cells. The viral structure includes EBV nuclear antigens (EBNA) 1, 2, 3A, 3B, and 3C; latent membrane protein (LMP)1 and LMP2; and RNA molecules produced by EBV (EBER1 and EBER2). However, the capacity of protein synthesis is higher and there is variation in the infection phases or types (such as permissible or non-permissible).6,7
Among these proteins, LMP1 and EBNA1 have a potential role in tumour pathogenesis. While EBNA1 expression is related to replication and maintenance of the viral genome during the dividing process, LMP1 mainly enables neoplastic cells to avoid apoptotic mechanisms;6 however, the complete sequence from EBV infection to EBV-related malignancy is still being studied. The number of B cells infected in the germinal centre from the lymph node may also be important. For example, comparisons between tonsils from Brazilian and German patients showed that the infection rate was similar but the number of infected cells was different, with higher numbers in the Brazilian samples (p<0.0007). Thus, there are other pathways to explain the EBV–neoplastic interaction.8
Tsai et al.9 investigated other factors that may explain the diversity of tumours related to EBV. Focussing on the differences between EBV strains, the authors showed that the strains B95-8, Akata, and GP202 induce more cellular growth than YCCEL1, SNU719, and M81. Akata and B95-8 had a significant tropism for B cells, which was not observed for YCCEL1 and M81; therefore, different EBV strains induce lymphoid tumours with distinct pathogenic aspects and there is a relationship between viral strain and tumour type and progression.9
In some individuals, the first contact with EBV produces a feverish state called infectious mononucleosis (IM). During this phase, a lytic and latent response involving T cells characterises the interaction between the patient and the virus.6 First described in the 19th century, IM is characterised by polyclonal expansion of infected B cells, which are triggers for a cytotoxic T cell response. The intensity of the CD8+ T lymphocyte response to EBV is key to controlling the infection.2 Patients usually have a fever with enlarged tonsils, cervical lymphadenopathy, reactive lymphocytosis, and variable splenomegaly. Lymphoid tissues show a mixed follicular, parafollicular, and sinusoidal monocytoid proliferation. In general, the paracortex has lymphocytes, plasma cells, and immunoblasts, sometimes in aggregates; it is also possible to find Hodgkin and Reed–Sternberg-like cells. Immunohistochemistry (IHC) is helpful because it demonstrates a reactive pattern with CD30+/CD15- cells in the middle of a polymorphous infiltrate.2
Following IM, lower viral levels persist in lymphoid tissues, especially in the oropharynx. This viral memory represents a risk of reactivation to the organism, which depends on specific factors.6 In this context, immunosuppression and chronic antigenic activation are possible triggers for the beginning of a new viral cycle and a neoplastic process.3 EBV may interact with key molecular pathways controlling the cell cycle, such as the NFκB pathway. The interaction between the virus and these pathways induces cytokine release with proliferative effects, as well as inhibition of apoptosis.2 Other findings are activation of MYC, BCL2, and NOTCH1, and induction of genomic instability.9
Viral latency is a strategy to avoid T cell immunosurveillance. EBV downregulates antigen expression, allowing a viral reservoir in memory B cells. In this context, there are four latency types:
- Latency Zero: Viral antigen is almost totally suppressed, despite viral genome being carried by B cells.
- Latency Type 1: There is an expression of EBNA1 in all virus-infected cells, which maintains replication of the episomal EBV genome.
- Latency Type 2: With the exception of EBNA2, many viral proteins are produced; LMP1 expression in the absence of EBNA2 confirms Type 2 latency.
- Latency Type 3: Unrestricted expression of nine latent genes, including EBNA1, EBNA2, EBNA3A-C, LP, LMP1, LMP2A, and LMP2B.
The importance of these latency types is that they only occur during an acute EBV infection or in the context of immunosuppression, situations in which there are strong cytotoxic T cell responses due to these viral proteins being highly immunogenic. Regardless of the latency type, levels of EBER1 and EBER2 RNA are constant, which indicate that they are a gold standard for the identification of EBV in tissue sections.2
EBV detection during routine pathology examinations is of increasing importance because the impact of the aetiology for diagnosis and prognosis has changed in recent years. Thus, the accuracy of detection is also important and the DNA and RNA integrity are valuable. For high-quality (mainly molecular) examination, fixation, post-fixation processing, and preparation of sections must be the priority of pre-analysis team members.10
Molecular tests are used for latent EBV infection diagnosis and include in situ hybridisation (ISH) and PCR. The membrane latency proteins can also be detected by IHC (Figure 1). In general, PCR sensitivity is higher than both ISH and IHC; comparisons of PCR and ISH analysis have indicated that both methods can produce similar results, depending on the probe mark and quality. Considering the similar results obtained, the cost of the techniques is often the determining factor and ISH has the best cost–benefit ratio so is recommended where possible. The use of IHC is also possible, but negative results must be interpreted with caution because the latency protein is not expressed in these cases. Thus, as discussed above, other proteins, including nuclear proteins, may be expressed.10
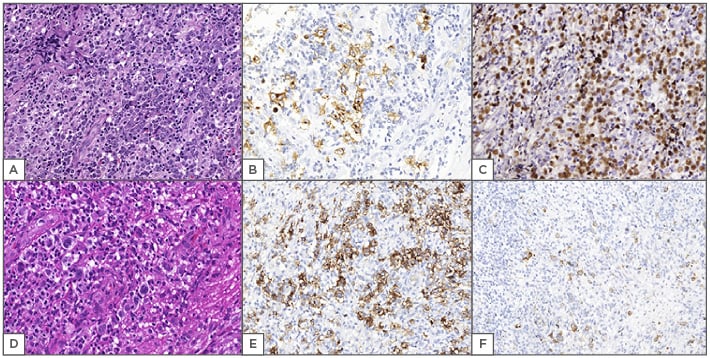
Figure 1: Examples of Epstein–Barr virus-positive diffuse large B cell lymphoma (A, B, C) and Epstein–Barr virus mucocutaneous ulcer (D, E, F).
A) (400x, HE stain) diffuse pattern with pleomorphic cells; B) (400x, CD30 antibody staining) neoplastic cells positive for CD30; C) (400x, CISH for EBV) nuclear staining using CISH to determine EBV infection; D) (400x, HE stain) EBVMCU is quite similar to HL: inflammatory background with atypical cells and sometimes Reed–Sternberg-like cells; E) (400x, CD30 antibody staining) other disease with atypical cells positive for CD30; F) (400x, LMP1 stain) EBV status is positive by immunohistochemical evaluations with LMP1.
CISH: chromogenic in situ hybridisation; EBV: Epstein–Barr virus; EBVDLBCL: EBV-positive diffuse large B cell lymphoma; EBVMCU: EBV mucocutaneous ulcer; HE: haematoxylin and eosin; HL: Hodgkin’s lymphoma; LMP1: latent membrane protein 1.
Another method for EBV detection is through plasma and peripheral blood mononuclear cell (PBMC) analysis. Kanakry et al.11 studied the clinical significance of PBMC detection, using viral quantitative real-time PCR of plasma and PBMC. Among patients with EBV-positive disease, the viral detection and quantification had greater specificity and sensitivity for diagnosis in the plasma than in PBMC. In the plasma, EBV-positive results were observed in 99% of patients.11 In addition, the authors demonstrated that the number of EBV copies was different in untreated EBV-positive lymphomas, EBV-positive lymphomas in remission, and EBV-negative lymphomas.11 Therefore, according to this group, the analysis of plasma for EBV detection represents a useful resource. In cases of EBV detection in histological samples, the correlation with plasma value is also important because the disease can have an atypical presentation and laboratory confirmation by different methods is a differential for clinicians.11
The objective of this article is to discuss EBV and lymphoid diseases, mainly the clinicopathological aspects. The main examples of lymphoproliferative disorders associated with EBV are classical HL, BL, diffuse large B cell lymphoma (DLBCL), plasmablastic lymphoma, primary effusion lymphoma, extranodal T and natural killer cell lymphomas, post-transplant B cell lymphoproliferative disease, mucocutaneous ulcers, and HIV-related B cell lymphoproliferative disease.2,5 In the new edition of the WHO classification of haematological and lymphoid tumours,12,13 two EBV-related diseases were highlighted due to changes in nomenclature after clinical observation and the description of a new entity: EBV-positive DLBCL and EBV-related mucocutaneous ulcer, respectively.2,5,12,13
EPSTEIN–BARR VIRUS-POSITIVE DIFFUSE LARGE B CELL LYMPHOMA
The WHO definition of DLBCL is a lymphoid neoplasm with diffuse pattern and neoplastic B cells characterised by nuclei larger than histiocyte nuclei. The classification evolution began with a morphological description and is now a molecular approach with important impact on prognosis of the most common non-HL worldwide. According to this evolution, subtypes of DLBCL were described. In the WHO 2017 classification,12,13 there are many specific subtypes, such as not otherwise specified (NOS); T cell or histiocyte-rich large B cell lymphoma; primary DLBCL of the central nervous system; primary cutaneous DLBCL, leg type; and EBV-positive DLBCL.12,14-16
Until 2008, EBV-positive DLBCL was called EBV-positive DLBCL of the elderly. The initial reports included patients with morphology of DLBCL and expression of EBER in malignant cell nuclei. The patients were elderly, had a poor response during therapy, and had a short survival time with standard combination chemotherapy.3 This subtype was known as a neoplasm that occurs in people >50 years old without any identifiable immunodeficiency or prior lymphoma. The tumour was understood in the context of immunologic deterioration related to the ageing process. Frequency of this subtype is estimated at 3–4% in the USA and Western Europe, and 8–10% in Asian countries.17
EBV infection is associated with immunosuppression and chronic antigenic activation, both of which are important components of the neoplastic process. In general, EBV-positive DLBCL patients have EBV latency Type 3 with expression of latent membrane proteins and nuclear antigens. Immunosenescence is characterised by thymic atrophy, T cell response dysregulation, anergic memory cells, and deficiencies in cytokine production. EBV may accelerate this process and provides the genesis of this subtype of aggressive lymphoma.3
Patients with this EBV-positive DLBCL have a worse survival rate than patients with EBV-negative DLBCL, with high International Prognostic Index scores, extranodal involvement, bone marrow infiltration, and an advanced clinical stage. From 2008–2016, studies demonstrated that EBV-positive DLBCL is not only found in individuals >50 years old;1,3,12 younger patients without immunodeficiency were also diagnosed with this pathology.12 In fact, older people have poor survival rates and therefore it may be the virus and other diseases that affect the elderly and immunosenescence.1,3
EBV-positive DLBCL is on the spectrum of activated B cell (ABC) diseases, which is a necessary classification after the WHO 2017 report.12 Using IHC markers, the lymphoma is classed as B germinal centre or ABC disorders. Initially, this process was a molecular classification supported by gene expression, but now it is an IHC classification based on algorithms by, for example, Hans et al.18 Detection by IHC results in a similar prognosis to gene expression and, in general, ABC patients have a poor survival rate. Recent articles and the WHO 2017 classification12 indicate that pathologists must perform an IHC test for calculation of Hans’ algorithms because the results are similar to the molecular findings; this methodology is controversial when using other similar algorithms and other markers.18-20
The monoclonality of Ig rearrangements is documented by analysis of the EBV terminal repeat copy number. In general, translocations involving MYC, BCL2, and/or BCL6 have not been found.19 On the other hand, copy number gains of these genes have been reported. EBV-positive DLBCL presents constitutive NFκB pathway activation and chronic active BCR signalling in a more pronounced way than EBV-negative patients with lymphoma.19 Other connections reported include high levels of expression of immune and inflammatory gene pathways in addition to NFκB, such as JAK/STAT, NOD receptor, and toll-like receptor signalling.19-21 The morphological presentation is an important disease indicator but does not influence the prognosis. The polymorphous subtype is more associated to EBV-positive DLBCL and has centroblasts, immunoblasts, and plasmablasts mixed with reactive cells such as lymphocytes, plasma cells, and histiocytes. Neoplastic cells are Hodgkin and Reed–Sternberg-like, often with geographic necrosis.3
Regarding IHC, CD30 expression is more common in EBV-positive DLBCL than in the other types of DLBCL;22,23 almost 40% of EBV-positive DLBCL patients are positive for CD30, but there is no cut-off for a positive definition and the intensity and extension staining are variable among patients.3,11 EBV-positive and CD30-positive patients have poor survival rates compared to EBV-positive and CD30-negative or EBV-negative and CD30-positive individuals.22,23 Considering viral proteins, 94% of cases have expression of LMP1, 10% have expression of EBNA1, and 28% have expression of EBNA2. Other B cell positive markers are CD20, CD19, CD79a, and PAX5. As mentioned, this subtype has an ABC phenotype with positive cells for IRF4/MUM1 and negative cells for CD10 and BCL6. Generally, CD15 is negative, but the differentiation with HL is derived from morphological aspects;12 in approximately 68% of cases, CD30 and CD15 are positive, making it difficult to differentiate from HL.20 However, a very important aspect for the differentiation is that extranodal HL are rare, representing an exclusion form for diagnosis.3 The WHO classification recommends ISH for EBER as a mandatory exam for an EBV-positive DLBCL diagnosis. In these cases, the positivity rates are 80% of the atypical cells.12 Expression of NFκB and pSTAT3 are more common in EBV-positive DLBCL than in EBV-negative lymphomas, which is evidence of the distinct gene expression profile of EBV-positive DLBCL.3 These lymphomas have an immune response in the context of a virus-induced inflammatory microenvironment with Ig heavy domain rearrangements and a B cell-activated phenotype. It is possible that the pathogenetic mechanism of this lymphoma in young people potentially has other aspects; for example, the EBV latency is more closely associated with immune evasion than a decrease in host immune competence. In this context, it is possible to compare EBV-positive DLBCL in young HL patients and EBV-positive patients. In both pathologies, genes related to the B cell receptor signalling pathway are supplemented by EBV-mediated activation of NFκB and upregulation of PDL2, for example, with immune evasion and poor prognosis.3,20
Treatment does not differ between DLBCL and the NOS subtype. Both subtypes are treated with the rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen.11,13 New therapeutic strategies include treatments targeting EBV and drugs against other pathways, with the objective of improving and/or modulating the immune response against EBV. One possibility found in the literature is the use of antiviral therapy combined with EBV lytic phase induction.11-13 This consists of the use of inducers of the lytic phase, such as methylase transferase inhibitors, histone deacetylase inhibitors, and proteasome inhibitors. Instead of typical antiviral therapies, such as those used for herpes virus, EBV requires a treatment that targets the latent phase, stimulating a lytic process.
Studies regarding conjugate antibodies, similar to rituximab, suggest brentuximab vedotin as an example of an anti-CD30 drug.21,22 Therefore, diagnosis using immunostaining is very important for CD30 in DLBCL, both for future treatment and for the correct diagnosis of EBV infection. Other options being studied are tyrosine kinase inhibitors and T cell therapy, stimulating cytotoxic T cells.3
EPSTEIN–BARR VIRUS-POSITIVE MUCOCUTANEOUS ULCER
In 2010, Dojcinov et al.24 described a series of 26 patients with immunosuppression due to medication, autoimmune disease, or immunosenescence, who had self-limited ulcers. All cases had similar pathological patterns characterised by Hodgkin-like features. The lesions were indolent, with good responses to conservative treatment. EBV status was examined through IHC using LMP1 and ISH using EBER. All individuals were positive for EBER, with good correlations with LMP1.24
The authors named the disease EBV-positive mucocutaneous ulcer (EBVMCU) and, in the 2017 WHO report, it was included as a category.13 Since the first series,24 22 new papers about this ulcer have appeared in the literature.25 The ulcer is described as occurring in patients with age-related or iatrogenic immunosuppression, often with a Hodgkin-like pattern and an indolent course, including spontaneous regression in some situations. There is currently no specific disease frequency recorded because this category is new to the scientific community. In general, it is classed as a lesion present in elderly patients at an estimated age >70 years; however, a history of immunological defects or changes has resulted in the diagnosis of patients at younger ages.13
More causes of EBV-lymphoproliferative disorders have been revealed in recent years and immunosenescence, primary immune deficiency, HIV infection, post-transplant setting, and the use of methotrexate and TNF antagonists have been suggested as possible aetiologies.24 In the context of HIV infection, oral ulceration is more common in the end stage of disease during AIDS and results in a decreased number of CD4+ T cells. Regardless of the similarities, in general, these patients have poor survival rates and EBV is not detected.26 In the cases of EBVMCU, for example, in elderly patients, the pathogenic point mutation induced the change in T cell response causing an accumulation of clonal or oligoclonal restricted CD8+ T cells with a functionality defect.12 Malignancies, haematological or otherwise, can also represent a setting for EBVMCU, independent of treatment status. The lesion may appear concomitant to a neoplasm, for example, an acute lymphoblastic leukaemia, reported by Vatsayan et al.27 as an indication to oncologists.28
In general, in EBV-lymphoproliferative disorders, the pathogenic focus is infection of the B cell. Approximately 39% of patients present findings of B cell rearrangements; however, a study by Dojcinov et al.24 showed 38% of EBVMCU patients had monoclonal TCR gene rearrangements, 31% had evidence of decreased T cell activity, and monoclonal Ig rearrangement was detected in 3 of the 26 cases. In addition to a B cell effect, there is also a deficiency in T cell control in EBVMCU patients.24
EBVMCU presents as ulcerated lesions, generally solitary and well demarcated in the oral mucosa, such as the tonsils, tongue, and buccal mucosa.5 Cases in the large intestine and rectum have also been reported27 and skin lesions are common, especially at sites such as the lips, arms, and torso. Lymphadenopathies appear near the ulcer infrequently, but systemic findings have not been observed.5
Histologically, ulcers have adjacent epithelial borders with pseudoepitheliomatous changes and acanthosis/epithelial hyperplasia. The inflammatory infiltrate presents a mixture of lymphocytes, plasma cells, eosinophils, and histiocytes. Large cells, such as atypical immunoblasts, and cells with two nuclei or irregular nuclei, similar to Hodgkin cells and Reed–Sternberg cells, have been detected.5 Apoptosis, angioinvasion, and necrosis may be present in the background.12 IHC examination shows the large, Hodgkin, and Reed–Sternberg-like cells are positive for CD20, ranging from weak to strong; the cells are generally heterogeneous. These cells are also positive for PAX5, IRF4/MUM1, and OCT2, and negative for BOB1, CD10, and BCL6. Those negative for BCL6 and CD10 and positive for IRF/MUM1 show the ABC phenotype. Generally, in these patients there is strong staining for CD30; CD15 may be expressed, but the subset of cells with this stain is not significant. Reactive T cells are presented in the background, with predominance of CD8+ T lymphocytes. Regarding EBV status, LMP1 is consistently positive, mainly in the large cells of immunoblastic type, and EBER is also positive in all types of cells, from lymphocytes to bigger cells, including the Hodgkin and Reed–Sternberg cells.2,5,12
In cases where HL is a consistent possibility, knowing about differential diagnosis is fundamental. Cutaneous or mucosal involvement by classical HL is the most probable possibility for these patients. However, careful consideration should be taken before diagnosis because HL manifestations outside the lymph nodes, with main involvement of skin and oral or gastrointestinal mucosae, are very rare. EBV-positive DLBCL is also a differential diagnosis. This neoplasm normally has CD15-negative neoplastic cells and a prominent diffuse pattern.5 In spite of the limited number of cases and series, different authors have affirmed the benign course of the lesions, with reports of spontaneous disappearance or good responses with reduction of immunosuppression therapy.12,23
In general, patients have a good prognosis with a self-limited disease. Other treatments include the use of rituximab in association with CHOP, local surgical excision, local radiation therapy, and, similar to EBV-positive DLBCL therapeutics, CD30-directed antibody therapy in the future.28,29 The therapeutic approach in EBV lymphoproliferative diseases, in general, is influenced by a variety of aspects, including the presence and/or the type of immunosuppression. Patients with age-related EBVMCU may receive aggressive treatments, such as chemotherapy, mainly with rituximab. Nevertheless, the current understanding of EBVMCU is that the disease is a self-limited condition in which a conservative treatment approach is sufficient.27-29 In fact, there is no guide for management of these cases because there are only a few cases published in the literature and opinions are based on self-experience only. Optimal clinical practice involves obtaining a detailed clinical history and detecting immunosuppressive conditions, associated to a biopsy analysed with the perspective of these differential diagnoses, in addition to a long follow-up with periodic evaluations of new lesions.
CONCLUSION
EBV and neoplastic disorders are key pathologies in the field of medicine, especially the pathology and oncology aspects of EBV infection. Epidemiologic data indicate this high importance, especially in Latin American, Asian, and African countries, where access to molecular methods for EBV detection in the context of public health is often difficult. For pathologists, it is important to learn about these diseases to better identify and investigate them. This article highlights some specificities about EBV-related lymphoproliferative disorders.
Firstly, when DLBCL presents atypically in Hodgkin or Reed–Sternberg-like cells, it is important to investigate EBV-positive DLBCL. One possibility is the inclusion of CD30 in the first IHC panel. If the cells stain for CD30, CD15 should be performed and EBV investigated with LMP1 or EBER. Secondly, lesions in mucosae with a morphological Hodgkin-like appearance should concern a pathologist. HL in mucosae is rare and so the lesion could be an EBVMCU. For this diagnosis, it is fundamental to have a good clinical indication of a possible immunosuppression status. Again, CD30, CD15, and LMP1 identification by IHC is a useful technique. It is also important to know that EBER is the best marker because it is expressed in all EBV latency types.







