Abstract
Thalassaemia is a chronic haemolytic anaemia that is endemic in the Mediterranean Basin. Extramedullary haematopoiesis (EMH) is a natural compensatory reaction involving several organs or tissues. This report outlines a case of dyspnoea due to bilateral dorsal paravertebral EMH, which was treated successfully with helical tomotherapy, a technique that combines intensity-modulate radiation therapy with image-guided radiation therapy. By the end of the first week of treatment, an increase in the haemoglobin value (up to 7.8 g/dL) and a remarkable reduction of dyspnoea were obtained, with haemoglobin values maintained at 7.8–7.3 g/dL without further blood transfusions. At 1-year follow-up, the patient was totally asymptomatic, with a complete resolution of dyspnoea and asthenia.
The therapeutic approach to EMH remains controversial because there are no pre-established protocols, with current treatments including serial blood transfusions, hydroxyurea, radiation therapy, and surgical decompression. This clinical case description reports how helical tomotherapy may be used as a valid and effective treatment for compressive atelectasis due to EMH. In fact, radiation therapy improved the general clinical condition and self-reported symptoms of the patient and reduced the size of EMH masses visible in the chest CT scan.
Key Points
1. Extramedullary haematopoiesis (EMH) occurs as a compensatory reaction in chronic haemolytic anaemias, including thalassaemia, in a variety of locations and can lead to the compression of neighbouring structures.2. Therapeutic management of EMH is controversial due to the absence of pre-established protocols, with current treatments including serial blood transfusions, hydroxyurea, radiation therapy, and surgical decompression.
3. Helical tomotherapy, which combines intensity-modulated radiation therapy with image-guided radiation therapy, was an effective treatment for compressive atelectasis due to EMH in this case, leading to a reduction in dyspnoea, reduction in the size of EMH masses, and an increased haemoglobin value at 1-year follow-up.
INTRODUCTION
Thalassaemia is an autosomal recessive haematological disorder that is characterised by defective synthesis of the globin moiety in haemoglobin (Hb), leading to chronic haemolytic anaemia. This condition is endemic in the Mediterranean Basin.1,2
Extramedullary haematopoiesis (EMH) usually occurs in almost all of the chronic haemolytic anaemias, as well as in myelofibrosis, polycythaemia vera, thalassaemia, sickle cell anaemia, leukaemia, lymphoma, or after bone marrow irradiation.2-9 EMH is a natural compensatory reaction involving several organs or tissues. The bone marrow of the vertebrae reacts to the increased demand for peripheral red blood cells by proliferating beyond the spongious bone of the vertebrae into the paraspinal regions. The most common involved sites are the spleen, liver, lymph nodes, adrenal glands, pleura, and spinal canal.1-9 The presence of haematopoiesis in these atypical locations can lead to the compression of neighbouring structures.1-3
The therapeutic management of EMH remains controversial, with treatments including blood transfusions, splenectomy, hydroxyurea, surgical decompression, radiation therapy (RT), or a combination of these.1-8 This case report describes a case of dyspnoea due to bilateral dorsal paravertebral EMH. The aim of this study is to demonstrate the efficacy of RT, in terms of local control and the improvement of symptoms, in cases of compressive atelectasis due to EMH.
CASE PRESENTATION
A 53-year-old female was diagnosed with non-transfusion-dependent β-thalassaemia (caused by a nonsense mutation at codon 39) when they were 2 years old, had received a splenectomy when they were 17-years-old, and was treated with blood transfusions until 2009. In 2009, hydroxyurea was started due to the occurrence of allogenic immunisation and self-immunisation. The first time the patient presented to the Radiotherapy Department of ARNAS Civico Hospital, Palermo, Italy, was in September 2019, where they were examined for anaemia (Hb: 6.3 g/dL) and dyspnoea, which was suggestive of compression of the lung parenchyma. A chest CT scan revealed several bilateral dorsal paravertebral masses with lung compression due to EMH (Figure 1). As a result, the decision was made to treat the patient with a total radiation dose of 2,000 cGy in 10 fractions (200 cGy/fraction) delivered over 2 weeks, after contouring T4–T12, the paravertebral haematopoietic masses, and the organs at risk.
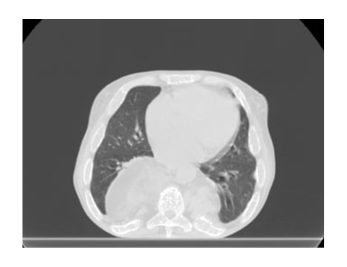
Figure 1: A chest CT scan, taken prior to helical tomotherapy treatment, showing paravertebral masses with lung compression due to extramedullary haematopoiesis.
Treatment planning was performed using the tomotherapy planning system. RT was delivered using helical tomotherapy (HT), a technique that combines intensity-modulate radiation therapy (IMRT) with image-guided radiation therapy (IGRT). This image-guided system (IMRT/IGRT HT) was based on the daily execution of a megavoltage CT scan prior to each fraction, in order to verify the accuracy of the setup. HT was used so that the effective dose could be delivered with a greater precision for the target, thereby saving the organs at risk as much as possible.
In just 2 weeks, the patient received encouraging results: an increased Hb value (7.8 g/dL versus 6.3 g/dL prior to treatment) and a remarkable reduction of dyspnoea by the end of the first week of treatment. After 1 month, dyspnoea was no longer observed and the Hb value was stabilised at 7.8 g/dL. A new chest CT scan showed a reduction in the size of the EMH masses with resolution of compressive atelectasis in the right lower lobe.
After 6 months, the patient had fully improved clinically, and a comparison of chest CT scans pre-IMRT/IGRT and post-IMRT/IGRT revealed a further reduction in the size of the EMH masses. At 1-year follow-up, the patient was totally asymptomatic. They had complete resolution of dyspnoea and asthenia, with Hb values maintained at 7.3–7.8 g/dL without further blood transfusions. The patient continued therapy with hydroxyurea. A chest CT scan revealed a regular bronchovascular distribution and that the size of the EMH masses was stable with no progression (Figure 2).
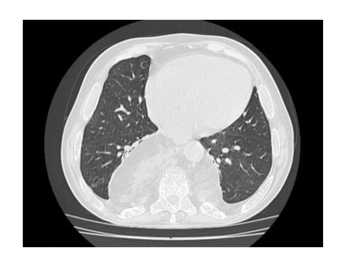
Figure 2: A chest CT scan, taken at 1-year follow-up from helical tomotherapy treatment, showing no progression in the size of the extramedullary haematopoiesis masses.
DISCUSSION
EMH is a common complication of ineffective haematopoiesis that occurs in many chronic haemolytic anaemias, including thalassaemia.2-9 EMH more frequently affects the liver, spleen, lymph nodes, epidural space, and paravertebral regions;2-9 while it less frequently affects the adrenal glands, kidneys, breasts, dura mater, adipose tissue, and skin.6 Moreover, EMH usually has a predilection for impacting the lower thoracic region, but the reasons for this are not yet completely understood.4,7-9 EMH is almost exclusively asymptomatic, but in rare cases it can compress the affected organ and lead to clinical signs.2,4,7,9 Spinal cord compression due to EMH in thalassaemia was first reported in 1954 by Gatto et al.10
Diagnoses of EMH are typically made based on a background of chronic haemolytic anaemia; MRI scans are the gold standard for showing EMH masses, especially the spinal cord compression related to these. In the absence of availability or if there is a contraindication to MRI use, a CT scan may be utilised.2,4,6,7 The treatment options available for EMH include blood transfusions, hydroxyurea, surgery, RT, or a combination of these.1-8 Due to the extreme rarity of this condition, direct comparisons between various treatment modalities are not possible.
Salehi et al.1 reviewed 56 cases of spinal cord compression due to EMH, of which some cases were treated with RT.1 Haemopoietic tissue is extremely sensitive to radiation and low doses can cause rapid shrinkage, so the advantages of this technique include its immediate availability and rapid clinical benefits. A few authors reported patients with spinal cord compression who underwent combined surgical decompression and RT with good results.2,7,11-13 In the cases reviewed, the radiation dosage used most frequently in different treatment protocols ranged from 1,000 cGY to 3,000 cGy.1,2
This case report presents a patient with respiratory symptoms due to the presence of paravertebral EMH masses. After RT, the patient showed a rapid and remarkable reduction of dyspnoea. There was a progressive reduction in the size of the EMH masses, and at 1-year follow-up, the patient was totally asymptomatic with no side effects occurring. Therefore, it was concluded that RT may be an optimal and safe therapeutic approach in such cases.
CONCLUSION
The therapeutic approach to EMH remains controversial because there are no pre-established protocols, with current treatments including serial blood transfusions, hydroxyurea, RT, and surgical decompression. This clinical case description reported how IMRT/IGRT HT can be used as a valid and effective treatment for compressive atelectasis due to EMH. The patient presented here showed rapid and remarkable results. They reported no more dyspnoea at 1-year follow-up and an increased Hb value, as well as a reduction in the size of EMH masses.