Abstract
Mycosis fungoides (MF) is the most common variant of cutaneous T cell lymphoma and frequently presents as early-stage disease with skin patches and plaques with an indolent course, but patients experience significant morbidity from itch and disfigurement. Around 30% of patients with MF present in the advance stages with skin tumours, erythroderma, and extensive nodal or visceral involvement. Sézary syndrome (SS) is the leukaemic cutaneous T cell lymphoma variant. The staging of MF or SS was revised in 2007 to include skin, nodal, visceral, and blood (tumour- node-metastasis-blood classification) to determine nine stages (IA–IVB). While most patients with early disease (Stages IA–IIA) have a good prognosis, 25% progress to advanced disease, with a poor life expectancy of around 3 years; however, some patients do survive for ≥10 years. Accurate staging is crucial since management strategies are stage-based, with skin-directed therapy recommended in early-stage disease and with no curative therapeutic options to improve symptoms and reduce skin tumour burden. In contrast, advanced-stage patients mostly require systemic therapy. Most treatments have only partial response rates, around 40%, and allogeneic bone marrow transplant may provide a more long-lasting therapeutic option for advanced patients.
Relevant prognostic factors within the tumour-node-metastasis-blood classification are discussed in this review and their relevance to overall IA–IVB staging and outcomes are debated. Several important prognostic features have been identified that may be used alongside staging to give further prognostic information. These prognostic features include age >60 years at diagnosis, large cell transformation of the skin, and raised serum lactate dehydrogenase levels, which could be developed into a prognostic index to identify patients at risk of progression and requiring more aggressive therapy. The PROCLIPI study, a prospective cutaneous lymphoma international study, has been ongoing since 2015 to collect such data, with the aim of developing a prognostic index for MF and SS.
INTRODUCTION
Mycosis fungoides (MF) is the most common primary cutaneous T cell lymphoma (CTCL), which comprise a heterogeneous group of non-Hodgkin’s lymphoma.1 Sézary syndrome (SS) is the leukaemic form of CTCL. The original staging system for CTCL was based on the tumour, lymph node, metastasis (TNM) system devised by Bunn and Lamberg in 1979.2 The TNM system, which was used to stage a wide range of malignancies, was revised jointly in 2007 by the International Society for Cutaneous Lymphoma (ISCL) and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer (EORTC) for MF and SS to include a blood stage, with an overall stage determined from the tumour-node-metastasis-blood (TNMB) classification, which stratifies patients into nine stages (IA–IVB) (Table 1). The early and advanced stages of MF are IA–IIA and IIB–VB, respectively,3 whereas SS is always an advanced disease from diagnosis and can be Stage IVA1–IVB. Management of MF and SS is stage-dependant, so accurate staging is essential for best management.4 Non-MF and cutaneous B cell lymphoma patients have a separate TNM staging system with no B category.5
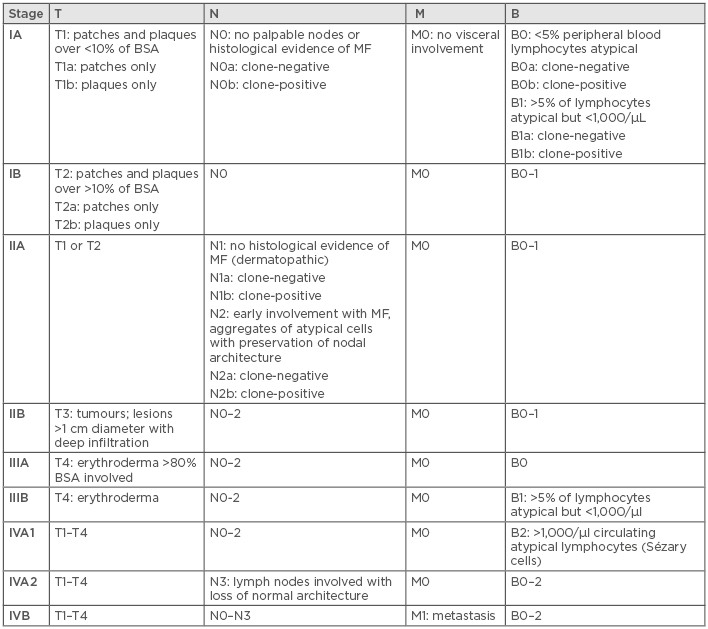
Table 1: Tumour-node-metastasis-blood (TNMB) classification for staging in mycosis fungoides and Sézary syndrome.
B: blood; BSA: body surface area; M: metastasis; MF: mycosis fungoides; N: node; T: tumour.
For a staging system to be clinically meaningful, it should have prognostic significance. While increasing stage classification often correlates with a worse survival rate, there are discrepancies, with some early-stage patients having rapidly progressive disease and others with advanced stages living >10 years.6-8 For example, those with Stage IIB disease may have a worse prognosis (median survival: 2.9 years) than those with Stage III disease (median survival: 3.6–4.6 years).6,8 Furthermore, patients with the folliculotropic variant of MF (FMF) Stage IB disease have a worse disease-specific survival rate at 10 years than those with Stage IIB disease.9 A large international study that included 1,275 MF and SS patients, staged according to the TNMB classification, found that, at diagnosis, raised serum lactate dehydrogenase (LDH) levels, large cell transformation (LCT) of the skin, and age >60 years were all significant factors for poor survival, independent of stage at diagnosis.8 This narrative review describes TNMB classification and discusses the recent advances in the identification of prognostic factors and development of prognostic indices for MF and SS.
TUMOUR-NODE-METASTASIS- BLOOD STAGING
The ISCL/EORTC TNMB staging system confirmed the general concept of the previous Bunn and Lamberg TNM staging system,2 but incorporated recent advances in tumour biology and newly developed diagnostic techniques. This included addition of a blood class (B), which splits erythrodermic patients according to blood tumour burden (B0–2) into Stage IIIA, IIIB, and IVA1. From 10 TNM categories there are now 21 TNMB categories: 7 skin classes (T1a–T4[3]), 7 nodal classes (N0–N3), 5 blood classes (B0a–B2), and 2 metastatic classes (M0 and M1). These classes are used to categorise patients into one of the nine stages from IA–IVB, as shown in Table 1.3 Although new T, N, and M subcategories were added to record the presence or absence of plaques (T1a/b and T2a/b) and T cell clonality (N1/2a/b and B0/1a/b), these are not used to determine disease stage.3
Tumour Classification
Skin involvement in MF can involve different cutaneous lesions defined as a) patch: any size lesion without induration or significant elevation above the surrounding uninvolved skin; b) plaque: any size lesion that is elevated or indurated; or c) tumours: any solid or nodular lesion >1 cm in diameter with evidence of deep infiltration in the skin and/or vertical growth. Erythroderma is defined as confluent erythema covering >80% of the body surface area (BSA). The type of skin involvement in MF and the amount of BSA involved are used together to define the T class from T1–4(3), where T1 is <10% BSA patches and plaques, T2 is ≥10% BSA patches or plaques, T3 designates tumours, T4 designates erythroderma, and T4(3) identifies erythroderma with tumours. Classes T1–2 are considered early skin lesions and T3–4 are advanced. The presence of patches only is denoted by T1a or T2a, and if plaques are present with or without patches, then this is denoted by T1b or T2b. Evidence of poikiloderma, follicular lesions, or ulceration should also be recorded. Ulcerative lesions are those with a significant loss of superficial skin, including the entire epidermis and some portion of the upper dermis, and may be plaque or tumour.
Additional information on skin tumour burden in MF or SS may be recorded using the modified severity weighted assessment tool (MSWAT), which is the preferred method for measuring skin tumour burden and is scored from 0–400.10 This skin scoring system provides a more comparative measure of skin tumour burden than T class, and it may be used to track skin involvement, notably during treatment. The BSA involved with patches, plaques, and tumours is calculated, usually using the palmar method where the patient’s palm equates to 0.5% BSA.11 Multiplication of patch x1, plaque x2, and tumour x4 produces a numerical value for MSWAT out of 400. Erythrodermic patients can similarly be scored by summation of the BSA involved with macular erythema (patch) and erythema with induration or oedema (plaque), while maintaining the ability to simultaneously track any tumours present.10 However, the prognostic significance of MSWAT has not yet been determined.
The PROCLIPI12 study is an observational study that has recruited nearly 1,000 patients from 44 specialist centres worldwide. The centres have collected prospective data at diagnosis, annually, and at stage progression, and these will be used to determine the prognostic significance of MSWAT, alongside other potentially important factors, with the aim of developing a prognostic index (PI) to preselect patients with a worse prognosis who require more aggressive therapies.12,13
Node Classification
Clinically abnormal peripheral lymph nodes (LN) are defined as ≥15 mm in any diameter or a firm, irregular, clustered, or fixed node regardless of size.3 Clinically enlarged or abnormal nodes should be corroborated by radiological imaging, with CT as the preferred technique. The largest peripheral LN or one that shows intense uptake on a fludeoxyglucose-positron emission tomography scan should be selected for biopsy and, if there are multiple nodes, cervical node biopsy should be selected above axillary and inguinal nodes. This hierarchical approach is used because cervical nodes have a higher chance of showing lymphomatous involvement.14-16
MF and SS LN status is denoted by N0–3, where N0 represents no clinically abnormal LN with no biopsy required. Clinically abnormal LN should be excised to determine the N class; an excisional LN biopsy is necessary to evaluate abnormal lymph nodes because core biopsy or fine needle aspiration do not provide adequate information on nodal architecture to define N3. Excisional LN biopsies are associated with morbidity typically from infection and in some instances may be deemed unnecessary by the treating physician; for example, this may be the case in patients with recurrent sepsis, previous LN biopsy, or when management will not be altered from the result. Clinically abnormal LN that have not been biopsied are classed as Nx. Excised LN histologically showing either no atypical lymphocytes or occasional or isolated atypical lymphocytes in clusters <7 cells are considered dermatopathic lymphadenopathy. LN classed as N1 (Dutch Grade 1 or National Cancer Institute Lymph Node [NCI LN] Grade 0–2) have early MF involvement that is defined by the presence of cerebriform nuclei >7.5 µm or aggregates of ≥7 atypical lymphocytes, and N2 defines LN with the nodal architecture preserved (Dutch Grade 2 or NCI LN Grade 3). Partial or complete loss of nodal architecture by atypical lymphocytes or neoplastic cells is scored N3 (Dutch Grade 3–4 or NCI LN Grade 4).3
It is advised that T cell receptor (TCR) gene analysis studies are performed on the excised LN. Clone-positive nodes are recorded as N1b or N2b and, correspondingly, clone-negative nodes are N1a or N2a. The use of ‘a’ or ‘b’ to record clonality is different to the T class, where ‘a’ denotes skin patches only and ‘b’ the presence of plaques. It must be appreciated that the N class only relates to the histology of the excised LN and does not give a measure of total LN burden. The PROCLIPI12 study involves prospectively recording the number of nodal sites (total nodal score) with enlarged LN at six peripheral sites and two central sites to score the patients LN burden from 0–8. The total nodal score is correlated with survival and N class may provide further prognostic information.12,13,15
Metastasis Classification
Visceral involvement with MF and SS is a well-documented, independently significant prognostic factor.8,17-20 Visceral disease is recorded as M1 and, independent of TNB class, is always categorised within the most advanced stage, Stage IVB.3 Visceral involvement tends to occur in late disease and <5% of patients present with Stage IVB.8,17-20 During the disease course, MF and SS may involve virtually all organ systems. More common extracutaneous systems are the liver, spleen, lungs, and the central nervous system, with the lungs being the most common.17 Visceral involvement is exceedingly rare in the absence of node or blood involvement and should therefore be questioned in these cases.3
Splenomegaly should be recorded as visceral disease, even without biopsy confirmation, when it is present on physical examination or documented radiographically (enlargement or multiple focal defects that are neither cystic nor vascular).3 A biopsy is not required because splenomegaly is rare in healthy persons and a spleen biopsy carries risks of bleeding. However, if lung abnormalities or other suggestions of extracutaneous lymphomatous involvement besides splenomegaly are seen on imaging, biopsy confirmation is usually recommended before categorising this as visceral involvement of MF or SS. Liver involvement may be suggested by clinical hepatomegaly, abnormal liver function tests, or radiologic tests (CT, fludeoxyglucose-positron emission tomography, and liver or spleen scan) and should be confirmed by liver biopsy.3 Cerebral lesions may occur and may not be amenable to biopsy. Visceral abnormalities could be from MF or SS, but infection or another unrelated cancer is possible since second malignancies are frequent in MF and SS.21-23
Blood Classification
The revised staging in 20073 introduced a blood classification (B0-2), in which blood involvement is determined using a manual Sézary cell count (on a peripheral blood smear) and scored as B0 (absence of significant blood involvement, ≤5% of peripheral blood lymphocytes are morphologically Sézary cells), B1 (low blood tumour burden: >5% of peripheral blood lymphocytes are atypical Sézary cells but does not meet the criteria of B2), and B2 (high blood tumour burden: ≥1,000/µL Sézary cells with positive T clone identical to the skin clone [relevant clone]). TCR clonality in the blood should be recorded as ‘a’ or ‘b’ alongside blood class in B0 and B1, but clonality does not alter the overall stage. For T cell clonality to be relevant it must be identical to the skin T cell clone (index clone) because blood clones may occur in the elderly population with unknown significance.3
The number of centres performing manual Sézary cell counts has declined in recent years because the process is highly subjective and requires considerable experience. Flow cytometry has therefore become the most popular alternative for the measurement of blood involvement in MF and SS.24,25 The 2007 staging paper acknowledges this decline and states that if Sézary cells are not able to be used to determine tumour burden for B2, then one of the following modified ISCL criteria may be used instead.3 The ISCL criteria state that B2 may be defined by flow cytometry in patients with a relevant T cell clone in blood as either a) expanded CD4+/CD3+ cells with a CD4:CD8 ratio of ≥10, or b) expanded CD4+ cells with abnormal immunophenotype including loss of CD7/CD26 (≥40% CD4+/CD7- or ≥30% CD4+/CD26-).26 However, no definition of expanded CD4+ cells was given. Furthermore, using the percentage of CD4+/CD7- or CD4+/CD26- cells, or the CD4:CD8 ratio, as opposed to absolute values, to define B2 has a disadvantage in that it detects patients with skewed CD populations but not necessarily a raised or high blood burden. In a paper by Olsen et al.,10 it was suggested that 1,600/µL can be used as an upper limit of normal for CD4 cells in the blood and an absolute count <250/μL CD4+/CD26- or CD4+/CD7- cells to define B0.10 A series of studies demonstrated that a very high blood tumour burden with >10,000/mm3 absolute Sézary cell count (H4 according to the suggested British classification) was associated with a worse poor prognosis.27-29 Recently, the EORTC Cutaneous Lymphoma Task Force published recommendations for B0–2 to be defined using absolute counts of either CD4+/CD7- or CD4+/CD26- by flow cytometry, where B0 is <250/μL (250 SI units), B1 is 250–<1,000/μL (250–1,000 SI units), and B2 is ≥1,000/μL (1,000 SI units), plus a relevant blood clone.24
Overall Stage IA–IVB
The MF or SS stage (Table 1) is the primary prognostic indicator and, although cases of MF or SS may relapse or remit with time and treatment, the stage cannot improve. Similarly, at stage progression, despite any subsequent improvement in TNMB, the stage cannot decrease. Therefore, the ISCL and EORTC recommend that, in addition to stage at diagnosis of MF or SS, the TNMB classification should be used to track tumour burden at any given timepoint to indicate the current and maximum tumour burden for an individual patient.3
DISCUSSION
The management strategy of MF or SS is decided according to clinical stage.4,30 Patients with Stages IA–IIA are deemed to have early-stage disease and are recommended for skin-directed therapy. Most of these patients have an excellent outcome and survive for 12–20 years or more.6,7,17-20 However, early-stage patients with FMF have been shown to have a poorer prognosis, more similar to tumour stage MF (Stage IIB).9,18 In addition, some patients with early-stage disease become refractory to skin-directed therapy and require systemic treatment, and around 25% of these patients progress to the advanced stages (Stages IIB–IVB).6 The advanced stages of MF and SS tend to have a poor prognosis and survival <4 years.6,8,17-20 Although some patients with advanced disease survive >10 years,6 Stage IVA2–IVB patient survival is almost universally poor and is commonly <12 months.8,17,18 Patients with advanced disease may require immunotherapy or chemotherapy and typically sequential treatments are given when a treatment response can no longer be measured. Treatment is frequently palliative in advanced MF or SS patients and decided on an individual patient basis, dependent on the presence of poor prognostic factors in addition to staging, but no algorithm exists and management of these patients varies between centres.4,30 Patients in remission may be offered an allogeneic stem cell transplant, but careful consideration is required because transplant-related mortality at Year 1 is significant (15–20%) and relapse rates are 39–51%; despite this, good responses to transplant and durable remissions may occur.31-33
Although T subcategories have been added to capture the different clinical presentations between patches and plaques (T1a/b and T2a/b), these are not used to determine the stage. However, an improved survival with patches versus plaques has been reported previously,18,20,34 while thick plaques are associated with a worse prognosis.35,36 Furthermore, the percentage of BSA involvement of the skin is only captured as <10% (T1; patches or plaques), ≥10% (T2; patches or plaques), or >80% (T4; erythroderma). The extent of skin lesions or skin tumour burden may be accurately tracked using the objective MSWAT scale, scored from 0–400. In time, the PROCLIPI study12 will determine the significance of patch versus plaque disease and skin tumour burden in MF and SS. TCR studies should be performed on skin to identify the index clone, which can then be compared to the blood TCR (and nodal if performed) to identify identical relevant T cell clones in the blood (or node) that are recorded as B0b or B1b (or N1b or N2b); however, T cell clonality in the skin is not included in the T class, which records plaques as T1b or T2b.
Skin tumours are always associated with advanced disease (Stages IIB–IVB) and there are conflicting results in the literature as to the survival differences between tumour stage (IIB) and erythrodermic MF (IIIA/B); some papers have showed a worse prognosis for tumour stage37 and others for erythrodermic patients,38 while similar survival rates for both have also been shown.18-20,36 The Italian Group of Cutaneous Lymphomas reported retrospective data on 1,422 MF patients; the only prognostic parameters selected by the multivariate analysis were the TNMB classification at first diagnosis and stage progression.19
Patients with early-stage skin lesions (patches with or without plaques) with either clinically abnormal nodes without a biopsy (Nx), dermatopathic nodes (N1), or early involvement of MF, but with preservation of nodal architecture (N2), are Stage IIA and considered early-stage disease. However, the prognosis is considerably worse than Stage I (Table 1), with N1 being associated with a higher relative risk of death compared to N0, and N2 being worse and more similar to N3 survival (Stage IVA2).18,20 Node subcategories were included to record clonality (N1a/b and N2a/b), where ‘a’ represents negative and ‘b’ represents positive clonality, following reports of a worse outcome in dermatopathic nodes with evidence of a relevant T cell clone, but do not alter stage.3
Visceral involvement or metastasis (M1) is rare at presentation of MF and SS and occurs in <5% of cases. It is almost universally associated with aggressive disease, which is preterminal and associated with a survival rate of <1 year. Apart from splenomegaly, histologic evidence of organ involvement is recommended for staging because the incidence of second malignancies and infection is common and may be treatable.21-23
Blood classification is recommended for all stages of MF and SS and was originally defined according to Sézary cell counts performed on peripheral blood smears;3 however, due to the expertise required for reporting Sézary counts and subjective results, it is not performed in all centres.24,25 Flow cytometry is now more commonplace and available at all expert centres in Europe, but it is frequently restricted to the advanced stages of disease and only 35% of centres perform flow cytometry on all disease stages.24 This leads to many early stages not receiving a blood class. Previous publications have used different definitions of blood class according to flow, and recommendations from the EORTC suggest that, for consistency and until further prognostic information is known, B0 is <250/μL, B1 is 250–<1,000/μL, and B2 is ≥1,000/μL. A very high peripheral blood involvement >10,000/μL has been shown to have further prognostic information and poorer survival than B2.28,29
Low-level blood involvement, as detected by a relevant T cell blood clone, should be recorded alongside blood class as polyclonal or clonal, but again this is frequently restricted to the advanced stages and performed at all disease stages in <50% of expert centres.24 This makes interpretation of the prognostic importance of blood class and clonality difficult and may bias results to association with later stage and worse prognosis.
The clinical relevance of a Stage B0a/b/B1a/b has not yet been proven, and the results of the PROCLIPI study12 will determine whether these are independent prognostic factors for survival. Recently, it has been shown by a European group that changes in blood tumour burden as determined by flow cytometry do not correlate with skin tumour burden.39 Staging of erythrodermic patients is affected by blood classification; those with B2 blood involvement with N1/2 and M0 are classed as Stage IVA1 or Sézary, as opposed to erythrodermic MF Stage IIIB (B1) or IIIA (B0), with different treatment recommendations.4,30 However, a recent large study of 1,275 advanced-stage patients found no significant difference in survival between Stages IIIA/IIIB and IVA1.8
Specific prognostic factors in MF outside staging have been reported over the past three decades, including improved prognosis with poikiloderma, association with lymphomatoid papulosis, and juvenile age of onset; on the other hand, age >60 years, FMF, and the histological feature of LCT have been found to convey a worse prognosis.40 The ISCL and EORTC recommend tracking patients with FMF or LCT to determine if either warrants a different staging system from classical MF and SS. A staging system that relates to prognosis is vital because this dictates the treatment for MF and SS.4,30
By combining clinicopathological features affecting survival, a PI may be developed to identify high-risk patients with diseases that have a wide range of survival rates. The development of a PI for aggressive non-Hodgkin’s lymphoma in 1993 has been widely used to stratify patients for treatment.41 Early attempts to develop a PI in MF and SS were not ratified in multicentre international trials.37,42-44 A cutaneous lymphoma PI for early-stage (Stage IA–IIA) and late stage (Stage IIB–IVB) disease from London, UK, included male sex, age >60 years, presence of plaques, FMF, and Nx/1 for early-stage disease, and male sex, age >60 years, N2/3, B1/B2, and M1 for late-stage disease.45 The predicted 10-year overall survival in the early-stage model was 90.3% (low risk) and 48.9% (high risk), and for the late-stage model was 53.2% (low risk) and 15.0% (high risk).45 A recent large multicentre study of 1,275 advanced-stage patients from 29 international centres identified four independent prognostic markers associated with a poor survival: Stage IV, an age at diagnosis >60 years, LCT, and raised LDH.8 Using these four parameters together in a prognostic model identified three risk groups across Stages IIB–IVB, with significantly different 5-year survival rates: low risk (68%), intermediate risk (44%), and high risk (28%).8 PROCLIPI12 has 956 patients currently enrolled from 46 international sites over five continents and has confirmed a male predominance of 1.6:1.0. This includes 680 early-stage (Stages IA–IIA) and 276 advanced-stage (Stages IIB–IV) patients and has found the median age at diagnosis of advanced disease is significantly older than early-stage patients, at 65 years and 57 years, respectively (p<0.0001). Furthermore, this large prospective study found the median time of MF-like lesions prior to diagnosis was 36 months in both early and advanced-stage disease, confirming diagnostic delay and suggesting that patients presenting with advanced disease are not undiagnosed early-stage patients. LCT has been recorded in 20 of the 680 (3%) early-stage patients and in 69 of the 276 (25%) advanced-stage patients (p<0.001). A total of 9% of early-stage and 30% of advanced-stage patients exhibited raised serum LDH at diagnosis (p<0.001).12
CONCLUSION
Staging systems are used to predict the likely outcome of disease and select appropriate treatments. Therefore, for a staging system to be clinically meaningful it must relate to likelihood of survival and response to similar treatments. The ISCL/EORTC staging of MF and SS provides useful prognostic information; however, some discrepancies exist and some patients with the early stages have a poor prognosis, while, conversely, others with advanced disease may live for 10 years or more. Also, Stage IIB may have a worse outcome than Stage III. By identifying patients at a high risk of progression, management may be tailored with the hope of improving survival, although no relevant biomarkers of disease activity or novel genetic alterations have been validated. However, several important independent prognostic factors are now well established in MF and SS, such as patch versus plaques, large cell transformation, FMF, raised serum LDH, and increased age, which may be developed into a PI to be used alongside staging to give further insights into the likely survival and help guide treatment choices. This is highly relevant because there are no curative therapies for MF and SS, and patients with a likely poor outcome should be selected for allogeneic stem cell transplantation, including some early-stage patients.
The PROCLIPI study12 is, and has been, collecting prognostic information prospectively at international centres in both early and advanced disease since 2015, but the data are not yet fully matured. At present there are not adequate prospective data on prognostic factors to deliver a PI for global use and there are not sufficient data to recommend a staging update, but it is anticipated this will be possible in the near future.







