Abstract
Percutaneous coronary intervention for chronic total occlusion (CTO) remains a challenging prospect for many interventional cardiologists. The treatment of these lesions is heterogeneous, as is the success rate. The aim of this review is to learn about how to approach these patients and lesions and discover the latest tendencies and research in interventional approaches in this field, as well as how to perform a useful pre-procedural approach, dual injection, lesion crossing, and modification to success. Finally, complications specific to CTO percutaneous intervention should be taken into account. Current guidelines, recommendations, and references to other significant articles which detail different aspects of management in patients with these complex lesions could be a useful guide for people beginning in this area. Algorithms of treatment, step approach, and proctoring are the current tendencies for CTO.
INTRODUCTION
Chronic total occlusion (CTO) is defined as the total obstruction of a coronary artery for >3 months. This duration has implications for the histopathological characteristics and the success rates associated with intervention.
Among patients diagnosed with coronary disease on angiography, CTO are seen in ˜20%,1,2 however rates as high as 50% can be found in patients who are receiving re-interventions, such as those who have previously had coronary artery bypass graft (CABG) surgery.1,3,4 Approximately 35% of CTO are currently treated by revascularisation (either CABG or percutaneous coronary intervention [PCI]). In the Canadian multicentre CTO registry, the majority of patients with CTO underwent medical treatment (64%) or were referred for CABG surgery (26%), while only 10% were referred for CTO PCI revascularisation.1 The referral rate of CTO PCI is between 1% and 16%, suggesting several issues:
- Patients are treated according to operator expertise (specific training) and institutional practice (high cost and resource utilisation) rather than clinical need5
- There are concerns regarding higher technical difficulty and perceived risk of complications compared with non-invasive treatment
- A lack of consensus regarding which patients would benefit from CTO revascularisation6
There are no randomised controlled data comparing recanalisation with medical therapy. The success of CTO PCI is largely dependent on the level of operator experience with different features of CTO,7 therefore step training of the operators in order to understand varied lesion specific characteristics is important.7-9
The rationale for the treatment of CTO has two key clinical decision points: first, whether to revascularise; and second, by which modality. Clinical need for revascularisation includes relief of the exercise-limiting symptoms of angina or dyspnoea, and also, prognostic factors such as improving regional left ventricular function.10 Figure 1 shows an algorithm to guide treatment decisions in these patients and lesions.
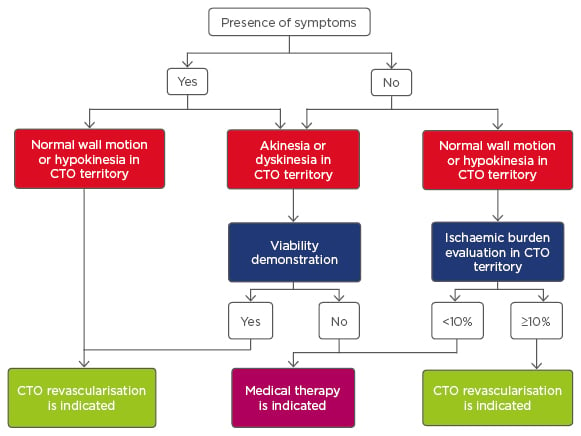
Figure 1: Algorithm of treatment in patients with chronic total occlusion.
CTO: chronic total occlusion.
Adapted from Galassi et al.11 with permission.
Discussions of optimal treatment are reflected in the evolution of the approach to CTO treatment in European12 and American13 guidelines as shown in Table 1. Since the publication of these guidelines, CTO PCI has undergone drastic improvements with success rates consistently >90% and major complication rates around 2% reported at several expert CTO sites.14-16 Taking economic aspects into account, it is uncertain whether PCI as an initial strategy would achieve a socially acceptable cost threshold, at any level of angina severity. However, a decision-analytic model suggests that CTO PCI is cost-effective in a patient population with severe angina symptoms. Relevant quality of life metrics should be employed prior to CTO PCI.17,18
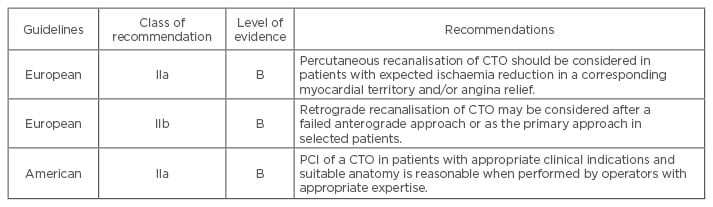
Table 1: Chronic total occlusion percutaneous coronary intervention in current guidelines.12,13
PCI: percutaneous coronary intervention; CTO: chronic total occlusion.
GENERAL ASPECTS IN CHRONIC TOTAL OCCLUSION LESIONS
Aetiology and Origin of a Chronic Total Occlusion
The development of a CTO could happen after a myocardial infarction; it has been described in 45% of patients without treatment, in 30% of patients after thrombolysis,19,20 and in 5–10% of patients after failed primary PCI or vessel reocclusion.21,22 However, the majority (˜60%) of patients with a CTO do not have a history of myocardial infarction.1 This could be due to the recruitment of collateral vessels to counterbalance the gradual progression to an occluded artery, limiting myocardial damage and resulting in mild or absent clinical symptoms.23
Histopathology
Anatomopathological studies of patients in whom a CTO was identified at least 3 months before, showed that thrombotic occlusion progressed over time from soft to hard. Long-term CTO, particularly without previous CABG, also have a high prevalence of negative remodelling.24 Hard intimal plaques are characterised by calcification. Although severity and extent of calcification and the collagen content of the intimal plaque increases with the duration of CTO, calcium is present in 54% of occlusions <3 months old. Within CTO lesions, microchannels are often observed and they might facilitate lesion crossing during CTO PCI. Microchannels mostly lead into the adventitia, small side branches, or vasa vasorum; however, they can also extend from the proximal to the distal lumen.25 These differences, along with an abrupt and tapering pattern of proximal and distal lumens, are likely to affect the success rate of PCI in CTO. Furthermore, the prevalence of the tapering pattern in the distal lumen was significantly higher than that in the proximal lumen, suggesting the advantage of a retrograde approach.24
Coronary Collateral Circulation
Anastomotic channels represent a native system for coronary arterial bypass allowing bidirectional flow. Collaterals therefore provide an alternative source of blood supply to the myocardium jeopardised by occlusive coronary artery disease, and they can help to preserve myocardial function in the setting of a CTO.26,27 Coronary collateral pathways are present in >70% of patients with either complete artery occlusion or coronary stenosis >90%.28
It is important to note that angiographically visualised collateral vessels do not always indicate myocardial viability. Thus a viability assessment should be undertaken before considering CTO recanalisation in patients with abnormal regional wall motion. In an invasive study using fractional flow reserve, <10% of collaterals provided a normal functional reserve during pharmacological stress.29 Cardiac magnetic resonance imaging with pharmacological stress testing, perfusion, and contrast enhancement is the optimal assessment to determine whether CTO PCI is indicated in in patients without severe symptoms or ischaemia.6,30 In patients with evidence of significant myocardial inducible perfusion defect and viability on cardiac magnetic resonance, CTO recanalisation reduces ischaemic burden, favours reverse remodelling, and ameliorates quality of life.31,32 Positron emission tomography/computed tomography,33 perfusion contrast echocardiography,34 and scintigraphy35 may be used as alternatives to magnetic resonance imaging.
From a technical standpoint, understanding the presence, quality, and location of collateral vessels is key to maximising the likelihood of CTO recanalisation procedural success. Dual injections of the CTO PCI target vessel and the donor artery or branch reveals the presence, size, and tortuosity of the collateral vessels and helps determine whether a retrograde approach is feasible.4 The presence of collateral flow does not seem to predict later restenosis or re-occlusion of the target vessel.36
Clinical and Epidemiological Characteristics of Patients with Chronic Total Occlusion
Patients affected by CTO sometimes show atypical symptoms; shortness of breath and exercise limitation are more frequently observed than typical angina.11 CTO are frequent in patients with relevant coronary artery disease (CAD), >50% have well-preserved left ventricular function, and ˜80% have no Q-waves in the CTO territory suggesting viable myocardium.1,2,37 In patients with diagnosed CTO the mean age is 66±11 years; they also have a higher prevalence of all cardiovascular risk factors and previous myocardial infarction than patients with CAD but not CTO.1
There are sex differences: 80% of patients with CAD and CTO are men,1 and women who receive a CTO PCI tend to be older, have higher rates of hypertension and diabetes mellitus, and are less likely to smoke compared with male patients. Moreover, female patients more often have a CTO located in the left anterior descending coronary artery, shorter and fewer blunt stumps and bridging collaterals than men, and are less likely to develop multivessel disease (MVD). However, after multivariable adjustment for known predictors, sex was not associated with CTO PCI failure.38
Indications of Treatment
There are three main reasons to recommend a CTO recanalisation:
- To relieve the exercise limiting symptoms of angina or dyspnoea, and, in moderately symptomatic patients, to resolve ischaemia detected by non-invasive stress testing
- To improve regional left ventricular function in the territory of the occluded artery
- To improve the prognosis of the patient as there is considerable risk of future progression of CAD in the remaining patent arteries10
CTO PCI is indicated when occlusion of an artery leads to angina, ischaemia,11,12 left ventricular dysfunction, and electrical instability, especially when the left anterior descending coronary artery is involved.6 Registries of patients with CTO showed that successful percutaneous revascularisation is associated with improved clinical outcomes: improvements in angina and quality of life,39 a potential improvement in electrical myocardial stability,32,40 a reduced need for CABG surgery,41 enhanced tolerance of future coronary events; increased left ventricular function.42,43 and in the context of complete coronary revascularisation, a substantial increase in survival.41,44-48
A rate of 10–12.5% of myocardium ‘at risk’ was recognised to be the cut-off point above which percutaneous treatment shows clear survival benefit for patients.2 However, in 2011 the STICH investigators reported that after correction of other prognostic variables, the presence of myocardial viability was no longer significantly associated with mortality. These findings bring into question whether left-ventricular ejection fraction recovery is completely dependent of myocardial viability, but might support the electrical or the ‘reserve’ hypothesis. In this hypothesis, patients with a CTO might be more prone to future cardiovascular events and have less reserve, especially during an acute occlusion in one of the remaining coronary arteries.49
PERCUTANEOUS TREATMENT OF CHRONIC TOTAL OCCLUSION
A detailed review of the techniques and materials available for CTO PCI are beyond the scope of this paper, however below is a summary of current recommendations and treatment algorithms which provide a useful introduction to the subject area.
Pre-Procedural Approach
The most widely used score to predict the overall likelihood of successful CTO PCI is the JCTO (Japanese Multicenter CTO Registry) score.50 It uses independent angiographic predictors of failure (each given one point): prior failed attempt, angiographic evidence of heavy calcification, bending within the occluded segment, blunt proximal stump, and occlusion length >20 mm. CTO were graded as easy, intermediate, difficult, and very difficult (JCTO scores of 0, 1, 2, and ≥3, respectively). In addition to crossing times, the JCTO score appears to correlate with long-term success.16
In addition, other independent predictors of failure were derived from the use of coronary computed tomography angiography (CCTA): occlusion length >20 mm, multiple occlusions, blunt stump, bending, and severe calcification in the CTO segment. Clinical predictors of failure included a previously failed PCI revascularisation and duration of CTO >12 months or unknown duration of occlusion. The use of CCTA is controversial because the indication of treating a CTO is a clinical one, and should not be made on the basis of the ease or difficulty of the case. As such, these scores are useful for identifying highly complex cases that should be referred to expert centres.
Although CCTA is generally seen as having limited clinical value, it offers assessment of:
- The length and three dimensional course of the occluded arterial segment
- The presence of calcium at the CTO
- The vessel size and vessel remodelling (either positive or negative)
- The quality of the vessel distal to the CTO
Patients presenting with an acute coronary syndrome require expedited recanalisation of the culprit vessel. Whenever a CTO in a non-culprit vessel is incidentally found, the patient should be given time to recover from the cardiac, vascular, and renal effects of acute coronary syndrome and PCI. A minimum of 1–2 weeks should elapse between treatment of the culprit lesion and planned treatment of a CTO. There is evidence that leaving a totally occluded artery untreated is associated with higher mortality at short and long-term follow-up post-ST-segment elevation myocardial infarction.2,12
Interventional procedures should be carefully planned; ad hoc angioplasty is not recommended. The operator should spend time examining the diagnostic films to decide if a unilateral or bilateral approach is required, to choose appropriate vascular access routes and dedicated equipment, and to guide catheter shape and size. Finally, remember that time constraint in the catheterisation laboratory is one of the factors linked with procedural failure.51 Proper catheterisation laboratory planning for a minimum of 2 hours is essential.2
The selection of the access route is dependent on the individual patient situation (e.g. severe peripheral vascular disease may mandate a radial approach). The radial artery could be used for either contralateral injection (5 or 6 Fr diagnostic catheters) or CTO treatment with 7 Fr sheathless guiding catheter.52 However, most experts use the femoral approach for the target CTO vessel (90% in Europe).2,12
When the distal vessel is mainly filled by retrograde collaterals, or there are bridging collaterals originating near the occlusion that are likely to have their flow impaired after wire-catheter advancement, contralateral injection is essential from the beginning of the procedure.2 When distal flow comes from a proximal branch of the occluded vessel, an 8 Fr catheter can be used to perform to perform selective injections in this branch with a microcatheter and cross in parallel the rest of the material to work in the occluded segment. Occasionally, single vascular access has been used to perform contralateral injections with different manoeuvres.53,54 Furthermore, single catheter antegrade injection should be avoided once dissection occurs in the antegrade space with subintimal entry as it may result in haematoma expansion.55 Bilateral access is therefore frequently needed.
Regarding a CTO there are two methodological approaches: the Japanese (proximal-to-distal) approach,11 and the American hybrid approach (Figure 2).51 The technical aspects, and pros and cons of each one were recently explained in an interesting review by Touma et al.56 In Europe and America the hybrid strategy has more followers, and it has also been used in CTO caused by in-stent restenosis.57,58
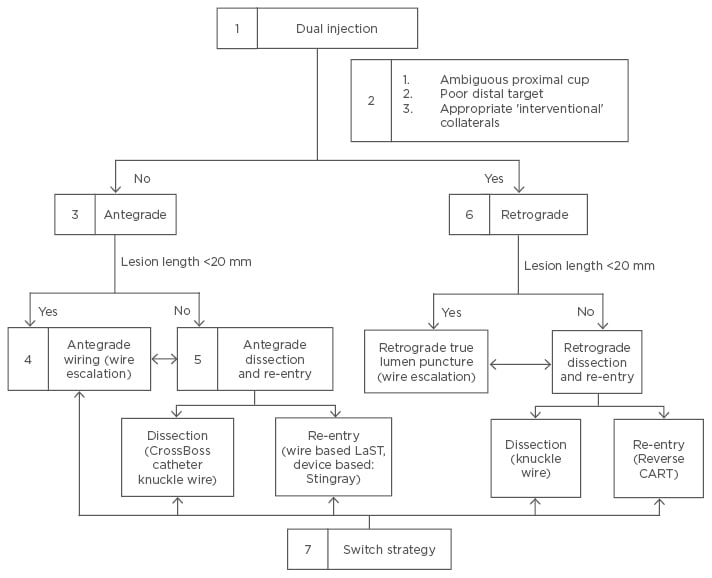
Figure 2: The American hybrid approach.
According to point 5 the ‘move the cap’ technique can facilitate antegrade crossing of CTO with
ambiguous or impenetrable cap using either a stiff guidewire (scratch and go), or a proximal balloon inflation to cause limited dissection to facilitate subintimal guidewire entry (balloon assisted technique). This entry in the subintimal space is followed by antegrade dissection re-entry lesion crossing.59 Stingray, Boston Scientific, Marlborough, Massachusetts, USA; Crossboss catheter knuckle wire, Boston Scientific.
CTO: chronic total occlusion; LaST: limited antegrade subintimal tracking; CART: controlled antegrade and retrograde tracking.
Adapted from Brilakis et al.51 and Touma et al.56
The hybrid approach is a systematic algorithm-led PCI strategy based on the identification of key anatomical features on baseline angiography.51 It introduces a structured approach, the main aim being to provide a reproducible, easily taught, and proctored technique for CTO PCI.60
This approach requires operator familiarity with all the available CTO PCI materials, techniques, and skillsets (antegrade wire escalation, antegrade dissection/re-entry with dedicated devices,61 and retrograde wire escalation and dissection re-entry). Flexibility with the various approaches, and timely change of strategies, is at the heart of the hybrid algorithm.
The limitations of disseminating the Japanese approach relate to cost and laboratory time. This approach is also highly individualised, making it difficult to teach and disseminate. Unique to Japan is the situation where patient preference results in low rates of CABG and this results in preferential treatment with PCI. Long case-duration may not be feasible when laboratory time is limited. Concerns have been raised over radiation and contrast volume during protracted cases.56
Lesion Crossing
In addition to strategies to facilitate lesion crossing and lesion modification, guidewire selection greatly influences the ability of an operator to successfully cross a lesion and wire the distal vessel true lumen. A variety of guidewires56 and supplementary manoeuvres specific to lesion subsets are available. In the JCTO trial, utilisation of advanced guidewires facilitated successful lesion crossing in 88.6% of CTO cases.50 It should be remembered that CTO-specific guidewires are more prone to enter subintimal channels, and may increase the risk of perforation. In order to increase the support to cross the lesion different techniques and devices can be used, including: anchor balloon support techniques, extension catheters, and microcatheters.
Lesion Modification
Once the lesion has been successfully crossed, further challenges related to lesion modification can arise. These modifications should be considered last resort measures where standard techniques have proved unsuccessful, taking into account the fact that in CTO they are all off-license settings;62,63 very high pressure non-compliant balloon dilation, balloon assisted micro-dissection, excimer laser coronary atherectomy, and/or high speed rotational ablation.
Stent Implantation
After successful procedures, implantation of drug-eluting stents (DES) is recommended. DES lead to an incremental 60% reduction in the relative risk of repeat revascularisation after CTO PCI.64 Clinical and angiographic restenosis rates are higher after DES of CTO compared to non-CTO, at least in part due to the diffuseness of disease, calcification, and number and length of stents required. Current generation DES may improve upon the results of first-generation DES after successful CTO recanalisation. The patency rate after CTO PCI at 69-month follow-up is approximately 90% for first-generation DES (sirolimus and paclitaxel-eluting stents), 97% for everolimus-eluting stents,65 and 94.3% for bioresorbable everolimus-eluting vascular scaffold (BVS).66 Before BVS can be recommended for routine application to CTO several issues must be addressed, these include their greater profile, tendency toward more recoil, and healing properties (particularly with subintimal implantation).67 Recently, similar clinical and angiographic outcomes in CTO revascularisation with zotarolimus and everolimus-eluting stents have also been discovered. Stent length, but not type of stent, was predictive for in-stent late loss and target lesion revascularisation rate.68
For the clinical community there are two interesting trials in progress: the EuroCTO and the DECISION-CTO,69,70 both of which compare PCI with DES and optimal medical therapy or optimal medical therapy alone. After these are complete we will have more evidence of the best option for treatment.
Complications and Outcomes in Chronic Total Occlusion Percutaneous Coronary Intervention
There are several general and specific complications related to this procedure such as contrast induced nephropathy (CIN) and radiodermatitis. The overall rates of adverse short-term clinical outcomes after percutaneous CTO intervention are declining (Table 2) and the current rates are approaching that of non-CTO PCI (Table 3).3
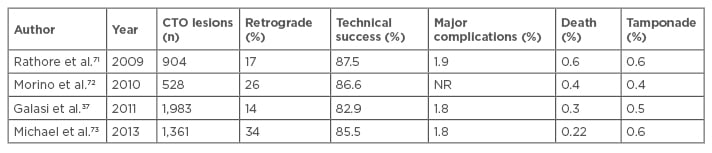
Table 2: Evolution of success and procedural complications in large registries of chronic total occlusion percutaneous coronary intervention.
CTO: chronic total occlusion; PCI: percutaneous coronary intervention; NR: not reported.
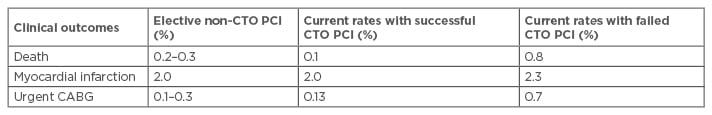
Table 3: Comparison of in-hospital clinical outcomes between chronic total occlusion and elective non-chronic total occlusion percutaneous coronary intervention.
CTO: chronic total occlusion; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft.
Adapted from Khan et al.3
Of paramount significance is the prevention of CIN during the CTO recanalisation procedure. Most operators would wish to keep dye load even in patients with normal advised to wait between 3 and 4 weeks.2 The risk of incidence of CIN was not different between the successful and failed CTO PCI group (7.2% versus 6.5%, p=0.45).44
The Mehran score74 suggested that ‘very high-risk group’ (≥16) classification and angiographic severe tortuosity were the predictors of CIN in CTO PCI. In daily practice, more careful hydration before and after the procedure, lower contrast volumes during the procedure, and precise follow-up after the procedure should be applied to these high-risk patients.2,74,75 Use of limited pre-procedural multislice spiral computed tomography, retrogradely positioned wires as markers (rather than using contrast injections), and intravascular ultrasound may all help to reduce dye load.2
Exposure to radiation is an important consideration; during CTO PCI it is necessary to make every effort to reduce radiation exposure (to the patient and the operator) and to document radiation exposure during the procedure. The procedure should be stopped when radiation reaches a maximum of 10 Gy. Strategies to reduce exposure could include using a frame rate for fluoroscopy of 7.5 pulses/s and changing projection.2
Urgent CABG in patients undergoing PCI for CTO could be needed in three common scenarios. Firstly, mechanical trauma to a vessel resulting in frank perforation and/or tamponade requires emergency cardiac surgery. Secondly, the inability to recanalise a CTO in the presence of MVD even in the absence of complications prompts a need for urgent bypass surgery in some symptomatic patients. Thirdly, in some patients with MVD and successful CTO intervention, post-PCI acute stent thrombosis can be managed with urgent CABG.
Complications unique to the retrograde approach may involve the occluded vessel (retrograde perforation/dissection), the collaterals (rupture/ haematoma of the septal or epicardial collaterals, perforation to the right or left ventricle, septal wire trapping, epicardial flow disruption with ischaemia), and the donor artery (potentially life-threatening dissection/thrombosis, spasm leading to ischaemia). The most common of these are related to the collateral vessels, but they tend to be minor and easily treatable in the majority of cases. However, perforation of epicardial collaterals, particularly in patients without prior open heart surgery, can be associated with tamponade and rapid haemodynamic compromise.
Although coronary perforations are common in CTO PCI (27.6%),71 most perforations are related to localised wire exit from the vessel architecture and are limited to angiographic evidence of contrast staining. As most perforations do not have serious clinical consequences, the risk of tamponade is low (0.3%).76 However, clinically significant coronary perforations can be associated with significant morbidity and mortality, with reported rates of death of 42%, emergency surgery of 39%, myocardial infarction of 29%, and transfusion of 65% in series not limited to CTO PCI.77 Management of perforations includes:
- prolonged balloon inflation across the perforation
- reversal of anticoagulation (not completely to avoid target and donor vessel thrombosis)
- covered stent placement, emergency surgery, or embolisation (coils, microsphere injection)
- pericardiocentesis
Experience in managing vascular and haemorrhagic complications is required since most CTO PCIs are performed through 8 Fr femoral sheaths, and the activated clotting time is kept in the 300 secs range during retrograde procedures.2 When feasible, the radial approach has fewer access site complications.78
Post-procedural deaths in patients can be broadly divided into two main categories: those directly related to procedural complications (perforation and tamponade), and those secondary to stent related complications such as acute stent thrombosis. However, deaths in patients with failed CTO intervention seem to be closely related to procedural complications as evidenced by higher rates of coronary perforation and cardiac tamponade (7.4% and 1.9% versus 1.16% and 0.2%, respectively) in this group. The risk of peri-procedural death as a result of a failed CTO PCI attempt ranges from 0.8–1.4%, which is still lower than the peri-operative mortality for CABG (1.76–3.5%).79
Outcomes following PCI CTO have been improving during recent years. Success rates in the treatment of complex CTO are 90–95%. They remain in the hands of a few dedicated expert operators, however the spreading of the hybrid approach algorithm, including the proctor’s help in difficult cases, is promising.60,80,81 These rates imply a huge knowledge about antegrade and retrograde techniques, dedicated materials such as catheters, guidewires, microcatheters, and catheter extensions, and how to manage complementary techniques such as CCTA and intravascular ultrasound.7 A recently published meta-analysis3 showed that, compared with a successful percutaneous CTO intervention, a failed CTO PCI is associated with higher rates of procedural complications: in-hospital mortality (1.44% versus 0.5%; relative risk [RR]: 2.88; p<0.001), peri-procedural major adverse cardiac event (8.88% versus 3.75%; RR: 2.25), myocardial infarction (3.17% versus 2.4%; RR: 1.35; p=0.032), and urgent revascularisation with bypass surgery (4.0% versus 0.5%; RR: 6.67; p<0.001).
Outcomes after CTO PCI may be enhanced by proper patient selection (resistant angina, large areas of ischaemia, and anatomic suitability for antegrade, retrograde, and/or subintimal recanalisation) and by reliance on expert operators using a flexible incremental approach to recanalisation.
CONCLUSIONS
There is evidence that successful CTO PCI results in improved long-term outcomes; however, a failed procedure is also associated with a higher risk of complications and adverse short-term outcomes. Therefore, patient selection for percutaneous CTO intervention should be individualised based on the risks of complications (probability of failed intervention) versus the benefits of improved long-term outcomes (in cases of successful PCI). Careful assessment of lesion morphology and other scores of risk, along with operator’s personal experience to deal with the specific lesion subtype is paramount before embarking on the procedure. Successful revascularisation correlates strongly with operator experience. For senior operators in high-risk complex CTO, proctoring and referral centres should be considered.








