Abstract
Prostate cancer, with its remarkably high prevalence, frequently creates clinical problems in terms of screening and diagnosis, as well as identifying the optimal window for treatment. Moreover, the prostate-specific antigen (PSA) blood test, despite being easy to perform, is routinely carried out without the patient’s informed consent. Although PSA-based screening for prostate cancer can reduce cancer-specific mortality, informed decision-making is mandatory; however, the clinician’s daily routine often neglects this critical discussion before performing a PSA blood test. This narrative review discusses the main questions regarding PSA screening and provides information on the epidemiological, clinical, and pathological aspects of prostate cancer.
INTRODUCTION
“Despite numerous clinical advances and innovations with hormonal palliation, age-adjusted death rates for prostatic cancer have not significantly changed in the past 40 years.”1 This is how Dr Gerald P. Murphy, leading cancer researcher and professor of urology, described the clinical situation of prostate cancer (PCa) in 1974.1 In 1987, the prostate-specific antigen (PSA) blood test was first described as a potential screening tool for PCa detection by Stamey et al.2 in a study that compared PSA to prostatic acid phosphatase. The results showed that PSA concentration was proportional to tumour volume and was a much better tumour marker than prostatic acid phosphatase.2 Stamey et al.2 also noted that less differentiated PCa with Gleason patterns 4 and 5 showed less positivity for PSA. A few years later, Catalona et al.3 published a landmark study showing that PSA was a useful adjunct to rectal examination for detecting PCa. Thus, a novel blood test able to detect PCa was introduced to clinical practice in the early 1990s. One must be aware that the prostate is embedded deep within the pelvis and apart from a digital rectal examination (DRE), which provides limited information on the consistence of the dorsal prostate part, no other clinical test was able to enhance diagnostics in PCa until MRI was developed for PCa screening.
Nowadays, screening for PCa is a controversial issue for several reasons.4 For example, there is ongoing scientific discussion regarding a lack of effect of PSA screening on PCa mortality reduction;5 however, many believe that PSA is a ‘simple’ blood test and very easy to perform. This belief has led to widespread use of this screening test, especially by general practitioners and urologists in the USA, and has resulted in a pronounced increase in PCa incidence.6 The high prevalence of PCa makes the discrimination between indolent and clinically relevant, potentially life-threatening PCa very difficult. Therefore, PCa is called a ‘two-faced disease’ by some.7 In addition, the results of the Prostate Cancer Prevention Trial (PCPT),8 which showed nearly 15% of men who were screened and had a normal PSA level had PCa, added additional uncertainty of the quality of this blood test in daily practice. Of note, the remarkable prevalence of PCa has been a well-known phenomenon for >50 years.9 This narrative review discusses the clinical and pathological aspects of PCa screening.
CURRENT EVIDENCE: HOW DOES PROSTATE-SPECIFIC ANTIGEN SCREENING AFFECT PROSTATE CANCER INCIDENCE AND MORTALITY?
A discussion on screening for a disease cannot take place without considering the prevalence and natural history of the condition. The prevalence of PCa is high and several autopsy studies have determined the relative frequency to be from 30–50%, depending on the age and the ethnicity of the patient group.10 Some of these studies were published as early as 1954.9 Table 1 summarises the prevalence of PCa according to age and ethnicity.10
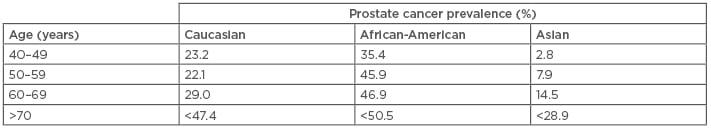
Table 1: Prevalence of prostate cancer according to age and ethnicity.10
Natural History of Prostate Cancer
PCa is known to have a long natural history. For instance, the SPCG-4 trial11 reported that approximately 30% of men not treated with curative intent died 18 years after randomisation (mean age: 65 years). Roughly 40% of patients developed distant metastases during this follow-up period and the use of androgen-deprivation therapy at 18 years was approximately 70%. Another study has shown that after a mean follow-up of 21 years, 16% of men died due to PCa12 (initial tumour stage: T2M0) and distant metastases occurred in 18% of patients at Year 32.13
In the pre-PSA era, Barnes14 investigated the natural history of patients with localised PCa who were treated conservatively. Half of the study participants survived for 10 years and 30% survived for 15 years. The most important prognostic factor affecting survival during this time was the grade of differentiation.15 Patients with poorly differentiated PCa were shown to have a shorter duration of natural history, with a 5-year cause-specific survival of 87% (well-to-moderately differentiated PCa), compared to 34% with poorly differentiated PCa.16 Importantly, one of the most relevant factors predicting overall survival is the competing medical hazard, as shown by Albertsen et al.17 Depending on the mode of detection, either clinically or screen-detected, PCa has a long natural history, which needs to be considered when counselling older patients for a PSA blood test.
Men undergoing PSA screening need to be aware of both the epidemiological and clinical background of PCa. Furthermore, men facing the decision of whether to undergo PSA screening need to understand the value of PSA-based screening in terms of the number of patients needed to be diagnosed to prevent one PCa death.18 Importantly, there is significant variation in the extent of shared decision-making in current PCa screening and treatment literature.19
ERSPC AND PLCO: THE TWO MOST IMPORTANT RANDOMISED CONTROLLED SCREENING STUDIES TO DATE
There are two major randomised controlled trials investigating the benefit of repeated PSA-screening on PCa mortality: the ERSPC20 and the PLCO21 studies. Table 2 summarises the characteristics of these two studies and compares them to the CAP study.20-22 In the screening groups of PLCO and ERSPC, the adherence to prostate biopsy was reported as 41–64% and 85%, respectively.23,24 It is important to note that study participants in the PLCO control arm received usual care; thus, >30% of study participants were pre-screened using a PSA test. Furthermore, in the PLCO control group, >80% of study participants reported PSA testing during study follow-up;25 however, the extent of further diagnostic work-up among screened participants from the control group (e.g., prostate biopsy and subsequent treatment) is not provided in the original study.26 When the estimated mean lead time as a proxy for PSA tests and further investigation among participants of the control group of both studies is compared, these values indicate a higher diagnostic work-up in PLCO than in ERSPC (estimated mean lead time: 3.1–3.4 years versus 0.7–1.7 years, respectively).27 Taken together, the results of the ERSPC study showed a reduction of PCa mortality, while those of the PLCO study did not, which is attributable to the high frequency of opportunistic screening in the usual care control group.
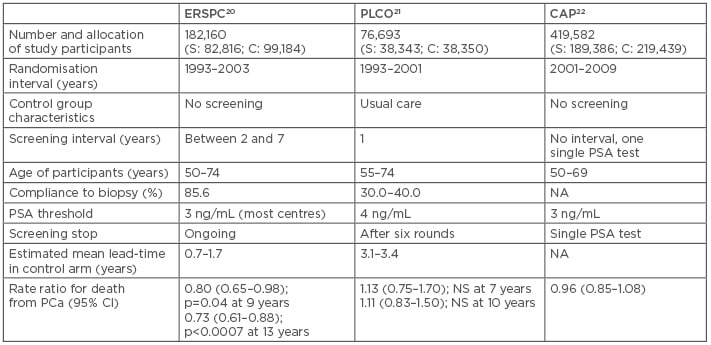
Table 2: Characteristics of three major prostate cancer screening studies.20-22
C: control group; CI: confidence interval; NA: data not available; NS: nonsignificant; PCa: prostate cancer; PSA: prostate-specific antigen; S: screening group.
Other screening studies for PCa have also been included in review articles or in the calculations for meta-analyses.28 For instance, a Swedish study29 randomised 1,494 participants to screening; overall, four rounds were performed, of which two were carried out by DRE only. However, DRE-only screening is insufficient to detect PCa at an early stage because only locally advanced PCa will be detected. This reduced the treatment efficacy in terms of mortality reduction. Moreover, in men with PSA levels below commonly used biopsy thresholds (e.g., 0–3 ng/mL, the PSA stratum in which most men will have a PSA value30), the positive predictive value of DRE for PCa detection has been shown to be very low at 5–30%.31 Despite its low number of participants and the insufficient screening method, data from this study are still used for meta-analysis calculations, remarkably altering the significance of high-level evidence.28
BIOPSY TECHNIQUE: INFLUENCE ON CANCER DETECTION AND OVERDIAGNOSIS
Many recent screening studies have used transrectal ultrasound (TRUS)-guided biopsy. With the results of two important studies,32,33 the evidence is clear that TRUS-guided biopsy is inferior to MRI-guided biopsy in terms of diagnosis and detection of clinically relevant disease.
The PROMIS study32 investigated whether multiparametric MRI (MP-MRI) could discriminate between participants with and without clinically significant PCa based on template prostate mapping (TPM) biopsy as a reference test. TPM biopsy was performed by sampling the entire prostate every 5 mm to accurately characterise disease status and reduce verification bias. A comparison of MP-MRI and TRUS biopsy in terms of sensitivity, specificity, positive predictive value, and negative predictive value on PCa findings was also performed.
On TPM biopsy, 40% (230 out of 576) of cancers were clinically significant, defined as having a Gleason score of ≥4+3 or a maximum cancer core length of ≥6 mm.32 For clinically significant cancer, MP-MRI was more sensitive (93%; 95% confidence interval [CI]: 88–96%) than TRUS biopsy (48%; 95% CI: 42–55%; p<0.0001) but less specific (41%; 95% CI: 36–46% for MP-MRI versus 96%; 95% CI: 94–98% for TRUS biopsy; p<0.0001).32 The authors concluded that if triaged men were screened using MP-MRI, 27% of primary biopsies could be avoided and 5% fewer diagnoses of clinically insignificant PCa would be made.32 If subsequent TRUS biopsies were directed by MP-MRI findings, up to 18% more cases of clinically significant cancer might have been detected as compared to the standard pathway of TRUS biopsy. MP-MRI could also reduce overdiagnosis of clinically insignificant PCa and improve detection of clinically significant PCa according to the study authors. MP-MRI could be recommended as a triage test before prostate biopsy in men who present with an elevated serum PSA.
The PRECISION study34 evaluated whether MP-MRI with targeted biopsy in the presence of an abnormal lesion was noninferior to standard TRUS biopsy in the detection of clinically significant PCa, defined as any Gleason score ≥7. A maximum of 3 areas that were suggestive for PCa were permitted to be chosen for targeted biopsy, with a maximum of 4 biopsy cores obtained per area, resulting in a maximum of 12 biopsy cores per participant. Standard biopsy was a 10–12-core TRUS-guided biopsy. Clinically significant PCa was detected in 95 men (38%) in the MRI-targeted biopsy group compared to 64 (26%) by standard biopsy. This study showed that MRI improved the diagnosis of PCa by enhancing the detection of clinically significant cancer, while also ruling out insignificant cancers following investigation of an abnormal PSA.
The results of these two studies, therefore, confirmed the value of MRI for patients with elevated PSA. However, neither the ERSPC nor the PLCO studies used imaging-enhanced biopsy techniques. The role of TRUS as an imaging tool has also been discussed in the literature. ‘Hypoechogenic lesions’ were considered pathologic by some authors but were found to be unspecific in clinical use. This makes it difficult for TRUS to be used to differentiate aggressive PCa and other inflammatory or benign tissues;35 nevertheless, recent research has indicated its potential role in the detection of aggressive PCa.36
THE EPIDEMIOLOGICAL ISSUE OF PROSTATE-SPECIFIC ANTIGEN SCREENING
Based on two large screening studies providing conflicting results in terms of disease-specific survival,24,26,37 several medical associations have changed their guidelines for practical management, including the United States Preventive Services Task Force (USPSTF).38 The change in USPSTF guidelines influenced PCa screening because there was a decrease in PSA testing in some countries, which led to more aggressive disease being missed at diagnosis.39 However, in comparison, testing in other countries may have increased. The crux is that the underlying prevalence of PCa10 frequently leads to well-differentiated, small PCa foci that have a low potential to harm the patient, leading to increased numbers needing to be detected (27 cases) to prevent one PCa death during 13 years of study follow-up.24 Due to the high PCa prevalence and untargeted use of PSA in primary care, in terms of opportunistic screening and subsequent prostate biopsy, the face of PCa has changed remarkably in recent decades, resulting in a considerable increase in PCa incidence.40 In most cases, PCa is not diagnosed clinically and is instead detected by needle biopsy at an early stage. In addition, since the introduction of MRI to clinical practice, these PCa foci can be detected visually.32,33 Emerging molecular imaging techniques will enrich the future of diagnosis and therapy.41
PROSTATE-SPECIFIC ANTIGEN SCREENING IN DAILY PRACTICE
Before the guidelines were changed, there was an increase in PSA screening during recent decades and the changing attitude to screening has led to a migration towards more cases of low-risk PCa.42 Frequently, patients ask healthcare providers for PSA testing. Although plenty of guidelines and recommendations on PCa screening exist, they seem not to be in accordance with screening policies of the past.43-45 It must be acknowledged that information about optimal retesting schedules is often sparse; in particular, retesting men most often does not rely on baseline serum PSA,43 although baseline values offer a powerful risk stratification.46 Importantly, the evidence for predictive properties for future PCa risk of a single PSA measurement has increased over the years.47-49 Moreover, the vast majority of a screened population will feature low PSA values at first screening. Therefore, in terms of PCa screening, healthcare providers carry a far-reaching responsibility in several ways. First, the patient is seeking advice as to whether to perform screening by a blood test. The patient generally has little knowledge about the screening performance of a test or about the exact prevalence of the disease. Second, PSA screening is being performed as a ‘single doctor screening modality’. For instance, screening for malignancies, such as colonic, lung, or breast, requires the collaboration with other disciplines, e.g., the gastroenterologist to perform a coloscopy or the radiologist to perform the CT scan, whereas PSA screening can be done by almost every doctor independently. Since most patients rely entirely on the doctor’s evaluation, a discussion prior to blood withdrawal regarding the diagnostic consequences, harms, and benefits of PSA screening is warranted. With regard to the normal PSA value, approximately 50% of men aged 50–70 years will have a PSA <1 ng/mL.46 These men will have a very low probability of developing harmful PCa during the next couple of years. If PSA is >3 ng/mL, risk calculators can help stratify patients.50
THE VALUE OF FAMILY HISTORY IN PROSTATE CANCER
A family history (FH) of PCa is a known risk factor for future PCa development. Meta-analysis results have supported the hypothesis that having a first-degree relative diagnosed with PCa is a significant risk factor for future PCa development in the index patient.51,52 This effect was particularly true for clinically diagnosed disease before the PSA screening era. Reported risk ratios vary from 2.5–3.4-fold higher than those of men with a negative FH, with a greater number of family members affected or younger age at diagnosis increasing this risk even further.53 A positive FH is, therefore, a key factor for identifying individuals who have an inherited predisposition to PCa. The goal of a FH inventory is to provide enough information for a risk assessment with respect to further investigations. However, the predominant part of the underlying data of those studies was gathered before or at the beginning of the PSA era. On the other hand, some noteworthy contemporary studies have failed to reproduce a correlation between a positive FH and PCa aggressiveness;54 for instance, the PCPT found PSA levels, DRE, and previous biopsy, but not positive FH, to be significant predictors for high-grade disease, despite a very high rate of positive FH of 17%.55 A limitation of this study was the high rate of positive FH and the randomisation of men with exclusively low baseline PSA. Moreover, a PCa diagnosis raises awareness of the disease for the patient’s family members as well as for their healthcare providers and, thereby, exposes male relatives to increased PSA testing and subsequent prostate biopsy. Additionally, a higher socioeconomic status was shown to be associated with detection of more localised but not metastatic PCa.56 Thus, a percentage of men with a positive FH diagnosed with PCa could be explained by increased screening behaviour. Box 1 gives a possible algorithm of how a screening visit might be carried out.

Box 1: Possible algorithm for a screening visit for prostate cancer.
PSA: prostate-specific antigen; TRUS-Bx: transrectal ultrasound biopsy.
CONCLUSION
Screening for PCa by a PSA blood test has been shown to reduce disease-specific mortality. However, because of the high prevalence of PCa, many clinically irrelevant tumours will be detected if a static cut-off value (e.g., 3 ng/mL) is applied. Therefore, 27 detections are needed to prevent 1 PCa death. Before the PSA blood test is performed, informed consent is necessary and the decision to screen should be well considered. Most men aged 50–70 years will have a low PSA value of <1 ng/mL.30,46 These men will also have a low risk of developing harmful PCa within the next couple of years. If a PSA value is found to be constantly elevated (i.e., ≥3 ng/mL), risk calculators should be applied. If the calculated risk is found to be increased, MRI fusion biopsy is the best biopsy method for these patients.








