Meeting Summary
Adjuvant chemotherapy (CT) is commonly recommended to breast cancer patients following surgery. However, not all patients benefit from it, and the intervention is associated with a substantial clinical burden, which also negatively affects quality of life. The aim of this symposium was to provide insights into the use of the 21-gene Oncotype DX® Breast Recurrence Score (RS) assay (Genomic Health Inc., Redwood City, California, USA) to optimise treatment decisions. The symposium started with an overview of the role of biomarkers in precision medicine in early breast cancer, provided by Prof Sparano, with a focus on recent developments in predicting CT benefit and assisting with the treatment decision-making based on the Oncotype DX® assay. CT is becoming a personalised medicine, comparable with oestrogen receptor (ER) expression testing and hormonal therapy, or human epidermal growth factor receptor (HER)2 testing and trastuzumab. Prof Sparano, the principal investigator of the TAILORx study, presented clinical trial and real-world evidence demonstrating a lack of CT benefit in approximately 80% of patients (those with RS results 0–25) and a substantial benefit in about 20% of patients (mainly those with RS results 26–100). This was brought into the perspective of clinical practice by Prof Penault-Llorca, who discussed the value of genomic assays versus classical pathological parameters and predictors of prognosis (e.g., age, ER and HER2 status, histological subtypes, Ki67 +/- mitotic index) and their associated risk of CT overtreatment and undertreatment. Prof Penault-Llorca also provided an insight into the lack of interchangeability of currently available genomic breast cancer tests. The symposium concluded with a presentation by Prof Nitz on CT decisions, specifically in node-positive breast cancer patients. Clinical and real-world data from large registries support CT decisions based on RS, independent of nodal status, to prevent overtreatment in daily routine.
Practice Changing Events: What Did We Learn?
Professor Joseph Sparano
A biomarker is defined as a characteristic that is objectively measured and evaluated as an indicator of normal biologic or pathogenic processes that may provide prognostic and/or predictive information that cannot be derived otherwise.1 They must demonstrate accuracy and reliability (analytical validity) and be statistically associated with the clinical outcome of interest (clinical validity).2 Additionally, their use in medical decision-making should lead to a change in treatment paradigms, which improves long-term patient outcomes (clinical utility).2 However, the latter is rarely demonstrated.
Biomarkers are increasingly used in breast cancer to guide therapy management and enable tailored treatment.3 Several developments have led to changes from a one-size-fits-all treatment approach, with surgery and radiotherapy for all patients, to precision medicine. In the late 1970s, the introduction of ER expression testing guided endocrine therapy selection. The late 1990s saw a breakthrough in treatment personalisation, with HER2 testing being used to guide the use of anti-HER2 therapy in metastatic breast cancer and subsequently in early-stage breast cancer. Likewise, the Oncotype DX® assay can be used to guide CT use in ER-positive HER2-negative early breast cancer.
Gene expression studies have demonstrated that breast cancer is highly heterogeneous, comprising biologically distinct tumour subtypes,4 and that prognosis and prediction of the benefit of CT are mostly driven by proliferation, ER, and ER-dependent genes.5 Several genomic assays have been developed on this basis. However, analyses have shown a 40–60% discordance in risk classification when other assays were compared with the Oncotype DX® assay, and fewer patients were classified as high-risk (and thus requiring CT) by the Oncotype DX® assay (Table 1).6-15
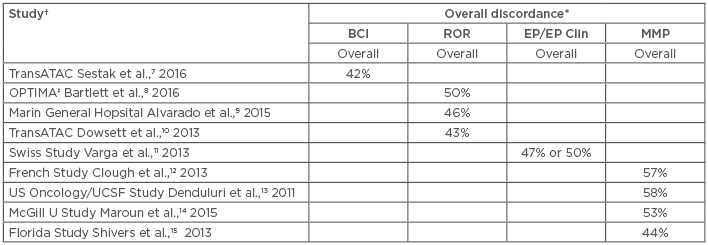
Table 1: Discordance between the Oncotype DX® Breast Recurrence Score and other assays.
*Overall discordance=any difference in risk classification between the RS assay and other; †Four studies did not include risk classification information appropriate for inclusion in this table; ‡Study used non-standard RS cut-off for the RS versus MMP comparison.
BCI: Breast Cancer Index; EP: EndoPredict®; EP Clin: EndoPredict® plus clinical features; MMP: MammaPrint®; ROR: Prosigna®; RS: Recurrence Score.
Adapted from Varga et al.6
The Oncotype DX® test estimates distant recurrence risk (DR) at 10 years and can preict CT benefit in women with hormone receptor (HR)-positive HER2-negative breast cancer, thereby assisting with systemic adjuvant treatment decisions.16,17 Specifically, this assay generates binary results in terms of prediction of CT benefit for node-negative patients, with those with RS 0–25 showing no benefit from chemoendocrine therapy over endocrine therapy alone, and those with RS 26–100 showing substantial CT benefit (Box 1).16,18-20
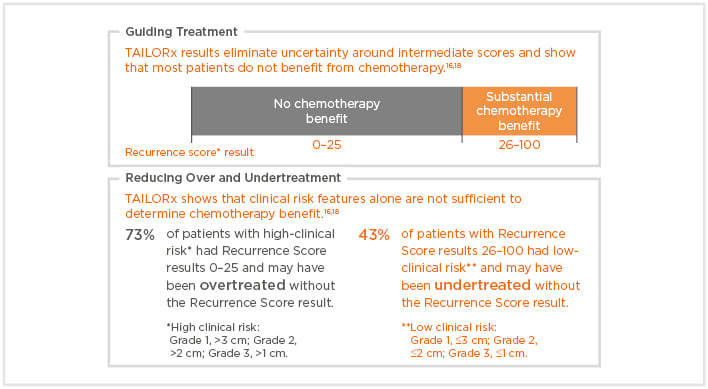
Box 1: The Oncotype DX® Breast Recurrence Score (RS) assay provides clarity for adjuvant treatment decisions.
The clinical validity of the Oncotype DX® assay has been reported in several trials. Prognostic information was demonstrated (level 1B evidence) in the prospective validation study NSABP-B14 using archived tumour samples from ER-positive node-negative breast cancer patients who had been followed-up for 10 years. Patients (n=668) had been treated with tamoxifen without CT and their RS results significantly correlated with DR rates.17
The predictive value for CT benefit was initially demonstrated with the two-arm validation study NSABP-B20 (level 1B evidence), in which ER-positive node-negative breast cancer patients (n=651) were randomised to receive either tamoxifen plus CT or tamoxifen alone. The study showed low DR rates at 10-year follow-up with endocrine therapy alone for RS 0–17 versus substantial CT benefit for RS 31–100 (relative risk [RR]: 0.26; 95% confidence interval [CI]: 0.13–0.53; decrease in absolute risk: 27.6%).16 Similar prediction of CT benefit was demonstrated for patients with RS 26–100 in the overall patient population of the NSABP-B20 trial (12% of whom were HER2-positive by reverse transcription-PCR)19 and in the subpopulation that included only HER2-negative disease.18,20 For patients with an intermediate RS result, CT did not seem to confer a benefit. Indeed, the wide CI for patients with RS 10–25 could not exclude a clinically important advantage.16 The TAILORx study was designed to address this question and has generated level 1A evidence that the Oncotype DX® assay can identify a large proportion of patients with HR-positive, HER2-negative, axillary node-negative disease who do not benefit from adjuvant CT.18,21 Thus, the trial provided an unprecedented level of evidence supporting the use of the Oncotype DX® test RS to guide CT use.18
Another prospective validation study, SWOG-8814, has provided level 1B evidence demonstrating the value of the Oncotype DX® assay to predict CT benefit also in node-positive patients.22 Data from the prospective WSG Plan B trial also provide evidence that the Oncotype DX® assay may be used to spare CT in patients with up to three positive axillary nodes.22,23
The clinical use of the Oncotype DX® test irrespective of nodal status has been confirmed in prospective registries and population-based analyses, including the Clalit registry study and the SEER database study, respectively.24–26 These consolidate data from patients tested with the Oncotype DX® test in Israel and the USA. In patients with limited nodal involvement, data confirm that those with low RS results who are treated with endocrine therapy alone have excellent clinical outcomes with low DR rates.24,25
The available evidence for the Oncotype DX® test has led to updated recommendations by the National Comprehensive Cancer Network (NCCN) and the German Institute for Quality and Efficiency in Health Care (IQWiG); both of these organisations support the use of the Oncotype DX® assay for guiding adjuvant CT treatment decisions in breast cancer.28,29
TAILORx Results: The Right Treatment for the Right Patient
Professor Joseph Sparano
Results of the aforementioned randomised adjuvant breast cancer treatment trial, TAILORx, were presented at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting.18 The trial was designed to address the challenge of integrating molecular diagnostic testing into clinical practice. The primary objective was to more precisely determine the effect of CT, if any, in patients at intermediate risk of DR (RS 11–25). Investigators used the Oncotype DX® test on every patient to quantify individual risk and assign treatment accordingly.18
The TAILORx trial used different RS groups (RS 0–10, 11–25, 26–100) from those used previously (RS 0–18, 18–30, 31–100).16-18 The RS result groups were selected based on the CI in the NSABP B-20 study to minimise the potential for overtreatment and undertreatment, while preserving prediction of CT benefit in patients with RS 26–100. When the NSABP B-20 data were reanalysed using the TAILORx RS ranges, the treatment effect of CT was similar to that of the original analysis.19 Consequently, TAILORx participants with RS 0–10 were treated with endocrine therapy alone; those with RS 26–100 received CT and endocrine therapy, as it had been previously demonstrated that these patients derive substantial benefit from CT and it would have been unethical to randomise these patients.17-19 To more precisely define the effect of CT with RS 11–25, 6,711 women (the primary study group) were randomised to receive endocrine therapy either with or without CT.18
Among patients with RS 0–10 who were uniformly treated with endocrine therapy, the DR-free interval (coprimary endpoint) at 5 and 9 years was 99.3% and 96.8%, respectively, indicating that these patients can be spared CT.18 In patients with RS 11–25, the invasive disease-free survival (coprimary endpoint) as well as the distant relapse-free interval, relapse-free interval, and overall survival (secondary endpoints) were similar between those treated with endocrine therapy alone and those treated with CT plus endocrine therapy, indicating little or no benefit of CT over endocrine therapy at 9 years.18 Patients with RS 26–100 presented a DR of 13% at 9 years with CT plus endocrine therapy, which is consistent with previous studies demonstrating similar outcomes in these patients: 12% DR at 10 years for patients treated with CT plus endocrine therapy versus 27% for patients with endocrine therapy alone in NSABP-B20 study.19
Altogether, the TAILORx primary analysis confirms that while patients with RS 0–25 do not benefit from receiving CT in addition to endocrine therapy, those with RS 26–100 derive substantial benefit from it.18,19 Additional exploratory subgroup analyses showed no significant interactions between CT treatment and the majority of the prognostic covariates examined, including tumour size (≤2 cm versus >2 cm), histological grade, and clinical risk category. This suggests that clinical pathological parameters do not predict CT benefit in the RS 11–25 arms.18 In these exploratory analyses, only age showed a significant correlation (p=0.004), since young patients (≤50 years) with RS 16–25 appeared to derive some benefit (1.6% and 6.5% absolute CT benefit in RS groups 16–20 and 21–25, respectively) from adding CT to adjuvant hormonal treatment.
Of note, patient characteristics in the TAILORx study were comparable to those in average clinical practice, as demonstrated by a comparison of the TAILORx study population with the SEER database.30 Similarly, the proportion of patients identified as deriving substantial benefit from CT (RS 26–100) was consistently in the range of 15–20% in clinical studies and registries.18,26,30-32
Overall, the TAILORx study adds to the body of evidence demonstrating that the Oncotype DX® assay can predict the magnitude of CT benefit in HR-positive, HER2-negative, node-negative breast cancer patients. The data show that the vast majority of patients with RS 0–25 do not derive a benefit from CT (study TAILORx, level 1A evidence),18 whereas patients with RS 26–100 (study NSABP-B20, level 1B evidence) derive a substantial clinical benefit from CT.16
Managing Decisions with Traditional Pathological Parameters and Other Tools: What is the Evidence?
Professor Frédérique Penault-Llorca
Classical prognostic factors and predictors of treatment response in breast cancer include age, histological subtypes, ER and HER2 status, Ki67 +/- mitotic index, vascular invasion, and tumour margins. Although useful, their clinical validity has not been systematically demonstrated. Furthermore, no factor has shown the ability to predict CT benefit; as a consequence, their use can result in CT overtreatment or undertreatment in >40% of patients.33 For example, the proliferation index Ki67 has demonstrated significant limitations due to a consistent lack of reproducibility. This has led the ASCO Tumor Marker Guidelines Committee to conclude that the evidence supporting the clinical use of Ki67 was insufficient to recommend its routine use.34
Considering the limitations of classical pathological parameters and strong clinical trial evidence on genomic assays, expert panels, such as the St Gallen International Expert Consensus, have endorsed the use of genomic assays in women with HR-positive breast cancer to avoid unnecessary CT.35
In the TAILORx study, unlike RS results, clinicopathological parameters (tumour size and grade) were found to have no predictive value for CT benefit in randomised arms.18 From a practical viewpoint, RS results thus contribute to reducing the risk of CT overtreatment or undertreatment. In the TAILORx trial, among the 2,812 patients with high clinical risk (Grade 1 and tumour size >3 cm, Grade 2 and tumour size >2 cm, or Grade 3 and tumour size >1 cm), 73% had RS 0–25 and would have been overtreated if a treatment decision would have been driven by classical pathological parameters alone. By contrast, among the patients with RS 26–100, 43% had low clinical risk (all other cases with known values for grade and tumour size) and would potentially have been undertreated without the RS result, effectively depriving them of the substantial CT benefit that patients in this RS range can experience.18
Genomic breast cancer assays other than the Oncotype DX® assay (e.g., MammaPrint® [Agendia, Amsterdam, Netherlands], Prosigna® [NanoString, Seattle, Washington, USA], and EndoPredict® [Myriad Genetics, Salt Lake City, Utah, USA]) have also been validated. However, the available tests are not interchangeable because of substantial differences in terms of genes selected, analytic and clinical evaluation, and risk assessment.
Additionally, as discussed, the tests have been shown to be highly discordant.36 The Oncotype DX® assay is the only test that has been used in HR-positive patients in randomised clinical trials with or without CT to assess the interaction between RS and CT benefit. The Oncotype DX® assay was proven to identify the small proportion of patients (with RS 26–100) who will overall benefit from CT, thereby minimising the unnecessary use of CT in the majority of patients (with RS 0–25).18 This is supported by high-level evidence,16,18,22,26,31 which contrasts with the paucity of clinical trial data on some of the other tests, particularly the lack of level 1A evidence for EndoPredict and Prosigna.21 Limited evidence supports the use of the Oncotype DX® assay and MammaPrint for predicting late recurrence, although this may not be a crucial issue given that high-risk patients usually receive CT.
On the basis of the available evidence, the Oncotype DX® assay has been incorporated into staging guidelines of the American Joint Committee on Cancer (AJCC), according to which patients with RS 0–10 are reclassified as Stage IA regardless of tumour size and grade parameters. NCCN guidelines also recognise the Oncotype DX® test as a predictor of adjuvant CT benefit in node-negative patients.28
Guiding Chemotherapy Decisions in Node-Positive Breast Cancer
Professor Ulrike Nitz
It has long been assumed that lymph node status was driving prognosis, based on evidence suggesting that overall survival decreases with greater nodal involvement.37 The TransATAC study also showed that nodal status is an independent predictor of DR. In the different RS risk groups, recurrence rates were very similar in node-negative and 1–3 node-positive disease but increase substantially with larger tumour burden (pN2).38 The study enrolled 2,929 HR-positive breast cancer women treated with anastrozole monotherapy. Of these, 1,231 were analysed for the Oncotype DX® assay to assess and validate prognosis value specifically in node-positive patients. It was validated that RS results from the Oncotype DX® assay strongly correlate with DR.38
Level 1B evidence supporting the predictive value of CT benefit in node-positive patients was brought by the SWOG-8814 study.22 This showed that in 413 node-positive patients randomised to endocrine treatment or CT plus endocrine therapy, RS result was a strong predictor of CT benefit for disease-free survival in patients with RS 31–100, and of no CT benefit in patients with RS of 0–17.22 Taken together, these data confirm that the Oncotype DX® assay is both prognostic and predictive for CT benefit, regardless of nodal status.
Prof Nitz, investigator of the WSG Plan B study, pointed to consistent results from this prospective randomised Phase III trial, which included 2,642 HER2-negative primary breast cancer patients who were node-negative (N0) at high risk or node-positive with 1–3 nodes (N1).23 The 5-year distant disease-free survival of patients with RS 0–10 treated with endocrine therapy alone (n=348) was similar in high-risk N0 and N1 subjects (97.7% and 97.9%, respectively).
Notably, this was comparable with the 5-year DR-free interval rate (99.3%) of N0 patients with RS 0–10 receiving endocrine treatment alone in TAILORx (n=1,626),30 confirming that these patients have good outcomes without CT, regardless of nodal status.
The prospective evidence from registries on the use of RS results to guide treatment choice in breast cancer is concordant with clinical trial findings and validates the clinical use of the Oncotype DX® assay in patients with micrometastases or positive lymph nodes.24,26 This indicates that patients with RS 0–17 have favourable clinical outcomes with endocrine therapy alone and can avoid unnecessary CT.
It is worth considering that a major clinically relevant discordance exists between the classical prognostic marker Ki67 and the RS result reflected in the Plan B study, with a significant proportion of patients having low Ki67 but high RS results, and vice versa. Therefore, a treatment decision based solely on Ki67 can potentially lead to an increased risk of CT overtreatment or undertreatment.27
The main implication for clinical practice, based on the available evidence, is that CT benefit is absent in early-stage breast cancer patients presenting with RS 0–17, low in those with RS 18–30, and substantial with RS 31–100. Further prospective data are expected from the ongoing RxPONDER study of women with node-positive, HR-positive, HER2-negative breast cancer (n=10,000).39 Those with RS 0–25 will be randomised to CT plus endocrine therapy or endocrine therapy alone and will be followed-up for up to 10 years. The primary endpoint is to assess RS predictive value of RS results for CT benefit. RxPONDER aims to consolidate the body of evidence supporting the use of the Oncotype DX RS assay in breast cancer patients and to define more precisely the magnitude of CT benefit in node-positive patients with RS 0–25.
Conclusion
The lack of accurate predictors of CT benefit has long been a limitation in HR-positive, HER2-negative, early-stage breast cancer, resulting in the inadequate use of CT in a significant number of patients and leading to an increased risk of overtreatment and undertreatment. Evidence indicates that the 21-gene Oncotype DX® Breast Recurrence Score (RS) test can identify with precision patients who can benefit from CT in addition to endocrine therapy and, in parallel, those who can avoid unnecessary CT and the associated burden. In women with HR-positive, HER2-negative, node-negative breast cancer, CT benefit varies with the combination of RS and age, and some benefit has been observed in patients ≤50 years with RS 16–25. In women with limited nodal involvement, CT can be avoided if RS is 0–17 because this patient group is better managed with endocrine therapy alone. Notably, in the TAILORx study, 73% of the patients in the RS 0–25 group had a high clinical risk, as assessed by classical pathological parameters, and 43% of the patients with RS 26-100 had low clinical risk. This suggests that CT is used unnecessarily in 73% of node-negative patients with high clinical risk, and that 43% of node-negative patients with a high RS result might be undertreated if decisions are based solely on a clinical risk assessment.








