Abstract
Ovarian cancer refers to a multitude of different cancer types originating from or involving the ovaries. Although it ranks third in gynaecological cancers, it is among the deadliest cancers in females. The prognosis mainly depends on early detection, but the majority of cases are diagnosed at advanced stages. Exact tumour delineation is crucial for individualised therapy planning. This review provides a practical update of the role of imaging in every phase throughout the course of this disease. The imaging technique of choice depends mainly on the clinical setting. Sonography remains the first-line imaging modality for cancer detection and is the most important for characterisation of adnexal masses. MRI is a valuable complementary imaging tool in sonographically indeterminate findings. For ovarian cancer staging, CT is considered an optimal imaging technique. CT renders all critical information for treatment stratification. It assists in surgery planning by displaying the load and the distribution of the disease and alerts to sites difficult to resect. It also renders critical information in selecting patients more suitable for medical therapy. In females treated for ovarian cancer, imaging is only recommended when there is suspicion of recurrence, where CT and PET/CT are most commonly used to confirm relapse and provide pivotal information for individualised treatment.
Key Points
1. Ovarian cancer is among the most harmful cancers in females and is generally diagnosed at a late stage, when the prognosis is poor. Moreover, despite optimal therapy, the relapse rate of ovarian cancer is as high as 70–85%. Almost 25% of females will relapse within 6 months and in most cases the cancer will recur within 2 years after completion of therapy.2. Imaging plays a pivotal role throughout the course of the disease: from the characterisation of ad- nexal masses to treatment planning, and confirmation of suspected ovarian cancer relapse.
3. Imaging gives crucial information for selecting candidates eligible for cytoreductive surgery. In this case, PET/CT is useful to demonstrate small or distant metastases. It is also optimally combined with MRI as it provides the single best modality for local surgery planning.
INTRODUCTION
Currently, ovarian cancer is recognised not as a single entity but as an umbrella term that refers to different malignancies arising from or involving the ovaries.1 The vast majority (85–90%) of ovarian cancers constitute of epithelial ovarian cancers (EOC) that mostly affect females who are of peri- and post-menopausal age. Recent advances in molecular and genetic analysis distinguish between five major histopathological subtypes of EOC, which differ not only in terms of origin, precursor lesions, prognosis, and molecular characteristics but also in emerging therapeutic implications.2,3
In fact, it is well established that premalignant precursors of high-grade EOCs, the most common type of ovarian cancer, and BRCA-associated cancers are arising not in the ovaries but in the distal fallopian tube.1
Although in the past four decades there has been an improvement in the 10-year survival rates from 18% to 35%, ovarian cancer is still among the deadliest cancers in females.4 This is mostly due to the fact that ovarian cancer is diagnosed at an advanced stage, when prognosis is poor, and, despite optimal initial treatment, it takes a fatal course characterised by serial relapses. Conversely, detection of ovarian cancer at an early stage or, ideally, of precursor lesions is associated with an excellent prognosis.
Imaging plays a pivotal role in females with ovarian cancer throughout the course of their disease, including for the characterisation of adnexal masses, treatment planning of ovarian cancer, and confirmation of suspected ovarian cancer relapse. From the beginning, disease imaging renders pivotal information for individualised tailored treatment.5 The selection of the appropriate imaging technique depends on various factors, but mostly on the clinical scenario. This review is focused on the value of the different imaging modalities used in assessing ovarian cancer.
EARLY DETECTION OF OVARIAN CANCER
Screening
Due to the lack of or only vague clinical symptoms, the vast majority of ovarian cancer is diagnosed at a late stage, when the prognosis is poor. The clinical impact of diagnosing invasive ovarian cancer or precursors such as borderline tumours early would be enormous.6 Early detection is the only way to achieve a high survival rate in females with ovarian cancer. Stage I ovarian cancer has an excellent 5-year survival rate of more than 90%.7 A recent comprehensive analysis of subtypes extracted from 28,118 ovarian cancers of the Surveillance, Epidemiology, and End Results (SEER) Program database showed that 39.2% of EOCs are diagnosed in Stages I and II. Of note, these tend to present the more indolent Type I ovarian cancers. In contrast, Type II (high-grade cancers and carcinosarcomas) tumours accounted for the vast majority of advanced stage cancers and were associated with a poor outcome independent of the stage.8
Unfortunately, there are no existing, effective strategies for screening ovarian cancer using imaging, rapidly emerging biomarkers, or a combination.9 At present, screening for females with a normal-risk of ovarian cancer is not recommended.9 Data from large screening programmes failed to show a survival benefit of females who were screened compared with females who were not screened.10,11 The rate of detected ovarian cancers was low, the performance in detecting Stage I disease was limited, and harms related to false positive testing were seen.
However, in females who are at high-risk of ovarian cancers due to BRCA mutations, a family history of ovarian cancer, or Lynch syndrome, semi-annual screening is recommended. Such a predisposition is estimated to occur in 10–15% of ovarian cancers. Carriers of the BRCA mutation have an increased life-time risk of ovarian cancer. It is estimated that carriers of the BRCA1 and BRCA2 mutations have an increased life-risk of 40–45% and 15–20%, respectively, by age 70 years.6,9 These females also tend to develop ovarian cancer in younger ages, but ovarian cancer is rarely found under the age of 40 years.9 In this population, transvaginal ultrasonography is generally accepted as the optimal imaging test for screening for ovarian cancer. Due to its high specificity, MRI can be offered for further characterisation of sonographically indeterminate masses. Thus, it may assist in reducing the number of surgical interventions if physiological ovarian masses or uterine fibroids are detected.12 Current evidence supports that prophylactic salpingo-oophorectomy reduces the ovarian cancer risk. It is recommended in the high-risk population, aged 35–40 years, or after the completion of childbearing.9
Prediction of Malignancy in Adnexal Masses
The accurate characterisation of an adnexal mass is essential for appropriate patient management.13 Likely benign lesions can be managed conservatively or by laparoscopic surgery, whereas females with malignant lesions will benefit from treatment by gynaecologic oncologists or in cancer centres.12,14 Advanced ovarian cancer usually requires radical cytoreductive surgery, followed by chemotherapy, or, alternatively, neoadjuvant chemotherapy, followed by interval debulking.15,16
Several imaging-based models for preoperatively assessing the risk of malignancy of an adnexal mass have been developed. These include the pattern recognition approach, e.g., the commonly used International Ovarian Tumour Analysis (IOTA) simple ultrasound (US) rules or other mathematical models developed by the IOTA, Risk of Malignancy Index (RMI), the Gynaecologic Imaging Report and Data System (GI-RADS), and the recently published Ovarian-Adnexal Reporting and Data System (O-RADS) risk stratification system.
Transvaginal sonography, combined with Doppler techniques, remains the mainstay for assessing adnexal masses. Due to its clinical utility and cost effectiveness, it has been established as the first-line imaging modality.17 It performs excellently in evaluating cystic adnexal lesions, which constitute the vast majority of adnexal masses. However, MRI is generally considered as a second-line, problem-solving modality and is particularly useful in complex or solid adnexal masses and when the clinical likelihood of malignancy is low.12 A systematic review demonstrated that the major advent of MRI is its high specificity to provide a confident diagnosis of benign adnexal lesions.18
Pattern recognition analysis, the gold standard for analysing an adnexal mass, is highly dependent on the level of expertise. Transvaginal sonography yields sensitivities of 85% and specificities of 90%, which might be even higher if they were performed in expert centres.19,20 The value of grey scale and colour Doppler US has been extensively analysed by the IOTA. Subjective assessment by highly trained clinicians in US performed equivalently to mathematical models such as the IOTA-simple rules models and logistic regression models.21 The combination of imaging techniques, usually with US, clinical features, and cancer antigen-125 (CA-125) levels is the basis of scoring in the RMI.
Recently, the O-RADS scoring and management system was introduced.13,22 This 5-point risk classification system for ovarian or adnexal masses was developed in close co-operation for US and MRI by the American College of Radiology (ACR) O-RADS Committee. A standardised imaging technique and terminology should be used for risk categorisation at initial diagnosis, as well as for the follow-up. In the US O-RADS score, ovarian masses are categorised based on their morphology and Doppler assessment. Incomplete evaluation (Score 0) and physiological ovarian follicles (Score 1) are separated from almost certainly benign (Score 2: <1%) masses, low risk of malignancy (Score 3: 1–<10%), intermediate risk (Score 4: 10–<50%), and high risk (Score 5: >50%) masses.22
US and MRI O-RADS were implemented to provide a standardised risk stratification system for ovarian and adnexal masses, which should serve as a consistent basis for analysis and reporting.13,22 O-RADS scoring should only be used in average-risk patients with no acute symptoms. It is also aimed at providing a guidance for management of adnexal masses in clinical practice. The integration of the risk score in the reports is ultimately intended to improve communication with referring clinicians by eliminating uncertainties in term usage and to assist in clinical decision making.13 However, clinical management directed by the treating physician supersedes management recommendations, based on imaging alone.13
Even in the hands of experts, 5–22% of adnexal masses will remain sonographically indeterminate or difficult to classify.23 Such masses typically exhibit the following sonographic features: large size, uni- or multi-locular, with solid aspects, irregular walls, papillary projections, and multilocular cysts. The vast majority of these lesions are benign tumours at histopathology, mostly cystadenomas, cystadenofibromas, and fibromas.23-25
To date, MRI is usually performed as a complementary imaging tool to further characterise adnexal masses that are indeterminate on US (Figure 1).12 The European Society of Urogenital Radiology (ESUR) guidelines recommend an algorithmic approach using basic and problem-solving sequences that will allow a confident diagnosis in the majority of cases, and thus assist in stratifying patients to the most appropriate treatment.12 Thomassin et al. introduced a 5-point risk score that combined morphologic pattern analysis, diffusion-weighted imaging, and perfusion analysis of solid tissue within an adnexal mass using time intensity curves.26,27 Recently, this MRI O-RADS score has been validated in a large European multicentre study. The results demonstrate a robust score, with sensitivities of 93% and specificities of 91%, for detecting malignant lesions in sonographically indeterminate masses, regardless of the level of radiological expertise.6,27 Data from this study also provided the evidence for the MRI O-RADS risk stratification scoring system (Table 1).
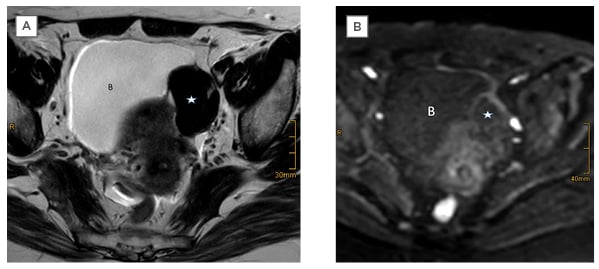
Figure 1: Left ovarian thecoma in a 55-year-old female.
Sonography showed an indeterminate solid mass. A solid tumour (star) is shown as separate from the uterus and adjacent to the bladder (B). MRI showed typical features of a benign tumour with low signal intensity on A) T2WI and B) DWI using a high b-value.
DWI: diffusion-weighted imaging; T2WI: T2-weighted image.
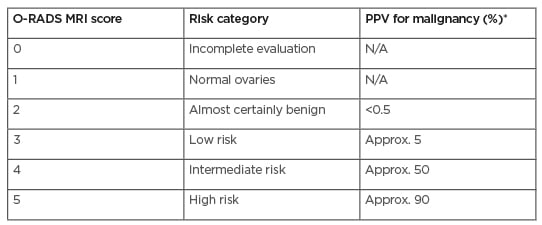
Table 1: Magnetic resonance Ovarian-Adnexal Reporting and Data System™ Scoring classification system.
The PPV values for malignancy include both borderline tumours and invasive cancers.
*Approximate PPV based on data from Thomassin-Naggara I et al.27
Approx: approximately; N/A: not applicable; O-RADS: Ovarian-Adnexal Reporting and Data System; PPV: positive predictive value.
Adapted from Reinhold et al.13
IMAGING FOR TREATMENT PLANNING IN OVARIAN CANCER
Traditionally, newly diagnosed ovarian cancer is surgically staged according to the International Federation of Gynaecology and Obstetrics (FIGO) or Tumour, Nodes, Metastases (TNM) staging classification. The 2014 updated FIGO system provides not only tumour stage categorisation but also incorporates information of the histological subtype and grade.28
Staging is usually performed during a staging laparotomy, including upfront cytoreductive surgery and is followed in most cases by taxane- and platinum-based chemotherapy.16 It has been established that cytoreduction to a cut off of 1 cm (optimal cytoreduction) is associated with increased survival in ovarian cancer.29 However, a trend towards ultraradical surgery, with complete resection of all gross tumour deposits, can be noted.30 The reported rates of optimal cytoreduction vary broadly from 15–85%, with high-volume oncologic centres achieving rates of optimal cytoreduction of up to 60–75%.31,32
The optimal treatment for the advanced cancer Stages IIIC and IV has long been a subject of debate.16,30 Recent data support that in advanced ovarian cancer, neoadjuvant chemotherapy renders equivalent survival outcomes but lacks the issue of high perioperative complications of ultraradical surgeries.16,30
CT has been established as an important tool not only for preoperative staging of ovarian cancer but also for providing pivotal information for management decisions.5 It is the mainstay of ascertaining the extent of the disease and exact depiction of distribution of the metastatic dissemination. It may also alert to sites that might be difficult to completely resect and where a multidisciplinary approach during surgery may be appropriate.33 Thus, it provides pivotal information for selecting between patients suitable for upfront cytoreductive surgery and those more likely to benefit from medical therapy prior to surgery, e.g., due to extreme tumour load or in patients unfit for surgery.34 When neoadjuvant chemotherapy is planned, an image-guided biopsy can be offered to confirm the diagnosis of disseminating ovarian cancer and to identify the histological subtype.33
Dissemination via the peritoneal cavity beyond the pelvis is typical for ovarian cancer and is found in more than 60% of females at diagnosis (Figure 2). The vast majority of these will present with high grade EOC. Solid or more diffuse peritoneal deposits are commonly found in the omentum, diaphragm, liver or spleen surface, the bowel, mesentery, and along peritoneal reflections. Perihepatic metastases (Stage III) present as a typical liver manifestation in newly diagnosed ovarian cancer. They usually present scalloping lesions with smooth margins along the liver surface but may sometimes invade the liver surface.35
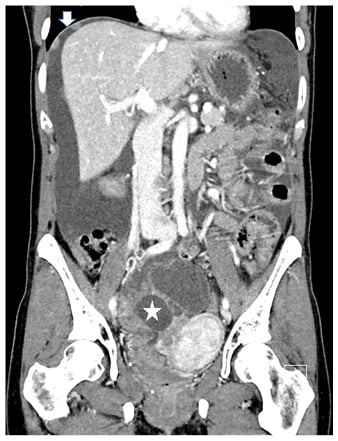
Figure 2: CT of advanced ovarian cancer.
Staging CT demonstrates a large solid and cystic pelvic mass (star) adjacent to the uterus. Large amounts of ascites are seen, as well as peritoneal implants at the dome of the right diaphragm (arrow).
Structured reporting of CT in ovarian cancer is a powerful tool of providing crucial information for therapy decisions in multidisciplinary meetings. The ESUR guidelines suggest not only defining the stage according to the FIGO or TNM classification but strongly recommend a management-driven, structured report that includes comprehensive information of the primary tumour, including the tumour burden and sites of the metastatic disease, as well as other information relevant for treatment planning.33 Potentially difficult to resect disease (not optimally resectable) should also be highlighted in the report. Although the clinical practice varies, these include tumour deposits of >2 cm in size in the root of the mesentery, gastrosplenic ligament, lesser sac, porta hepatis and falciform ligament, and suprarenal or supradiaphragmatic lymphadenopathy.34 Various tests have been developed to predict the likelihood of optimal cytoresection.31,32,36 Major determinants include clinical risk factors, CA-125 levels, and imaging tests, most commonly using CT. However, the clinical utility of such standardised prediction of resectability could not be proven.24,31,32
To date, preoperative staging of ovarian cancer remains a CT domain. It is widely available, reproducible, and provides all relevant information for staging in a short examination time.33 The reported accuracy for all stages ranges from 70–90%, with an overall sensitivity in detection of peritoneal implants of 85–93%.34,37 Limitations, however, include small volume peritoneal disease (<5 mm) and sites such as the bowel surface and the mesentery. PET/CT is not routinely used for initial staging of ovarian cancer but may be useful as an adjunct test to inconclusive CT findings or in case of contraindications for contrast media.33,38 Although MRI performs similarly for staging and is superior to CT in the visualisation of small peritoneal implants, it is still regarded as a second-line imaging modality.33,38 Apart from costs, this is mainly attributed to technical issues and a much longer examination time. MRI is recommended when radiation exposure or its superior soft tissue contrast capability is an issue, e.g., in pregnancy or in young females presenting with presumed borderline tumours and if fertility preservation is considered.38 Similarly, the role of fluorodeoxyglucose-PET/CT remains that of a problem solving imaging test, that is particularly useful in advanced ovarian cancer or as an alternative to contraindications of contrast-enhanced CT. Whole body MRI has shown excellent results and performs comparably to PET/CT for assessing metastatic disease within and outside the abdominal cavity.39 As this imaging technique is emerging from research to clinical application, it may become a central management tool in newly diagnosed ovarian cancer.
PREDICTION OF TUMOUR RESPONSE IN OVARIAN CANCER
Neoadjuvant chemotherapy prior to cytoreductive surgery is a treatment option in selected patients with advanced EOC. Clinical, routine serial CA-125 assessments and CT serve as the mainstay for response assessment during this therapy. CT is usually performed as a baseline study and after 3 cycles of chemotherapy to determine eligibility for cytoreductive surgery. Alternatively, in insufficient response, medical treatment is continued.
Assessment of the change in tumour load is a critical feature in patients undergoing clinical trials. Here, response has traditionally been assessed by serial CA-125 monitoring and standardised quantification of the tumour using Response Evaluation Criteria in Solid Tumours (RECIST), mostly in CT. In ovarian cancer, however, application of the RECIST criteria is challenging due to the diffuse peritoneal spread in most cases of EOC and the problem of defining target lesions.40
A recent, large multicentre study conducted in patients undergoing neoadjuvant chemotherapy showed that response criteria using only CA-125 or RECIST were limited to predict optimal cytoreduction.41
CA-125 is the currently best-established biomarker in ovarian cancer. However, a multitude of potential novel biomarkers are under investigation. Moving forward to individualised treatment of ovarian cancer, research activities include comprehensive molecular profiling of tissue biopsies and of tumour DNA circulating in the blood (liquid biopsies).
Prediction of tumour response prior to therapy is also an area of ongoing research, utilising the functional imaging techniques of PET/CT and MRI.40 In one study, apparent diffusion coefficient quantification obtained by diffusion-weighted MRI showed differences between the primary tumour and peritoneal metastases, reflecting inter-site tumour heterogeneity and potentially resulting in different biological effects.42
Radiomics and radiogenomics data render information beyond the morphological tumour manifestations. Radiomic signature of the primary tumour and deposits based on preoperative CT may provide information as a prognostic imaging biomarker in high-grade ovarian cancer.43 The role of both intra- and inter-site heterogeneity in ovarian cancer has been addressed by Vargas et al., who showed that CT radiomic features of tumour heterogeneity were associated with a poorer outcome and incomplete surgical resection.44 In the future, the combination of radiomic features and clinical data may allow for the development of predictive models of resectability or of tumour progression.45
IMAGING OF THE TREATED OVARIAN CANCER
Despite optimal therapy, the relapse rate of ovarian cancer is as high as 70–85%.46 Almost 25% of females will relapse within 6 months and, in the majority, the cancer will recur within 2 years after completion of therapy.47 In advanced ovarian cancer, the 5-year overall survival rate differs across the different subtypes of EOC.48
When ovarian cancer has relapsed, it is a treatable but rarely curable disease. In general, relapse introduces a chronic and consecutively lethal stage of the disease, with reported survival rates ranging from 12–32 months.49 Survival after the relapse is related to several factors including chemosensitivity, the time of progression-free interval after therapy, and the number of recurrent lesions. The standard of care for recurrent ovarian cancer is systemic platinum-based chemotherapy or subsequent line therapies. Secondary cytoreductive surgery is not routine but may be performed in selective patients.49 This individualised approach depends on the size and site of the recurrent disease and is usually based upon a multidisciplinary conference decision.50 Cytoreductive surgery for relapsed ovarian cancer is mostly performed in the localised disease at its first recurrence or after a long disease free-interval.50,51 Patients with a prolonged platinum-free interval (>6 months) and those with an isolated or limited volume of disease and a positive Arbeitsgruppe Gynäkologische Onkologie (AGO) score are likely to benefit from secondary surgery compared with those with a shorter progression-free interval or large volume disease.51
Although there is lack of evidence that routine imaging after completion of primary therapy improves survival in ovarian cancer, in clinical practice CT is commonly used for surveillance in females in clinical remission.52 Major gynaecological guidelines, however, advise against routine imaging in a patient treated for ovarian cancer.49,50,52 Surveillance is recommended by clinical assessment and CA-125 monitoring for 3–4 months in the first 2 years and in a 6 months interval for Years 3–5. Imaging in a female treated for ovarian cancer is only recommended if recurrence is suspected, e.g., in rising CA-125 levels or in clinical symptoms suspicious of relapse.49
Ovarian cancer usually relapses in the abdomen or the pelvis, with recurrence found in the surgical bed in up to 50% of patients.53 Peritoneal carcinomatosis is the typical manifestation of metastases and is seen in approximately 75% of cases.54 Less common manifestations of metastases in treated ovarian cancer are the lymph nodes, lungs, pleura, and liver parenchyma.53,54 Unusual and rare types of recurrence including isolated distant, central nervous system manifestations, bone, skin, or soft tissue metastases tend to occur late in the disease.55
CT has been widely used for assessing suspected recurrence in patients treated for ovarian cancer. Complementary PET/CT is mostly performed in patients with unremarkable or equivocal CT findings but rising tumour markers. A systematic review reported that contrast-enhanced PET/CT has a pooled sensitivity and specificity of 93.94% and 93.80%, respectively, for detecting recurrent disease.56 Apart from whole body assessment, advantages of PET/CT include the depiction of small size metastases or metastases at sites difficult to assess with CT, e.g., of the bowel surface, mesentery, or subtle lymphadenopathy. However, microscopic peritoneal recurrence or lymph node metastases with a diameter of less than 5 mm are also beyond the detection of fluorodeoxyglucose-PET/CT.57
In patients treated for ovarian cancer, imaging renders pivotal information for tailored treatment planning in multidisciplinary boards. It ascertains the presence of suspected recurrence and demonstrates their site and tumour load. It also provides other important information such as potential complications of treatment.54 Imaging also renders crucial information for selecting candidates eligible for cytoreductive surgery. In these patients, distant metastases as well as irresectable local disease have to be excluded. In this scenario PET/CT is superior to CT to demonstrate small or distant metastases.58 It is optimally combined with MRI, as this presents the single best modality for local surgery planning, e.g., the pelvic sidewalls or other important structures.5 Advances in the management of ovarian cancer recurrence are to be expected when PET/MRI will become more widely available in clinical practice.







