The more widespread use of inhaled corticosteroids (ICS) for the treatment of asthma following guideline recommendations 27 years ago1,2 delivered huge benefits to many patients in terms of improved disease control and fewer of what were then called ‘asthma exacerbations’. These benefits have been repeated many times as low-dose ICS have become freely available in low and middle-income settings;3 however, what ICS have not done is ushered in a new golden age, wherein asthma is no longer a problem because of this new treatment. In fact, far from asthma being a thing of the past, it remains an ever-present threat of death and morbidity, with reduced quality of life during childhood, the loss of adult working life, and problems continuing into old age. There is also no sign of any improvement likely in the near future.4 Why is this? We were previously charged with leading the Lancet Asthma Commission5 addressing this question head on. We suggested that the lack of a new age of asthma therapeutic could be attributed to the following reasons:
- The success of ICS treatment has made the medical world complacent; we now believe that anyone can treat asthma and putting ICS in the tap water is the answer to all problems.
- We have come to accept that almost anyone who has any respiratory symptoms probably has asthma and no objective testing is needed; patients should just be prescribed ever higher doses of ICS until the symptoms disappear.
- We have complacently regarded asthma exacerbations as a trivial inconvenience, readily reversible with oral prednisone, and requiring no other special action.
Nothing could be further from the truth than these current and widely held beliefs. Taken point by point, the consequences have been:
- We have grossly over-treated many patients with asthma, having failed to heed the lessons of Dr Harry Morrow-Brown, who clearly demonstrated that only asthma with sputum eosinophilia responded to steroids.6
- Asthma has been grossly over-diagnosed and thus has become trivialised.
- Symptom relief has been regarded as the main goal of treatment with ICS, but complete symptom control is not attainable in many patients, despite good control of airway inflammation.
- The dose–response curve for ICS plateaus at relatively low dosage levels and the addition of further medications often fails to bring benefits, usually because the diagnosis is wrong or the originally prescribed medications are not being taken.
- The scientific community has failed to appreciate the fact that these acute deteriorations are asthma attacks not exacerbations. The acute deteriorations carry a huge risk of further attacks and death, as well as having long-term consequences,7 and should thus be considered a red-flag never-event. This thinking has not entered the collective consciousness, despite repeated reports of asthma deaths.8
Against this background, with asthma outcomes stalled (Figure 1) and asthma mortality unchanged over many years, the Lancet Commission was conceived. There were seven major recommendations:
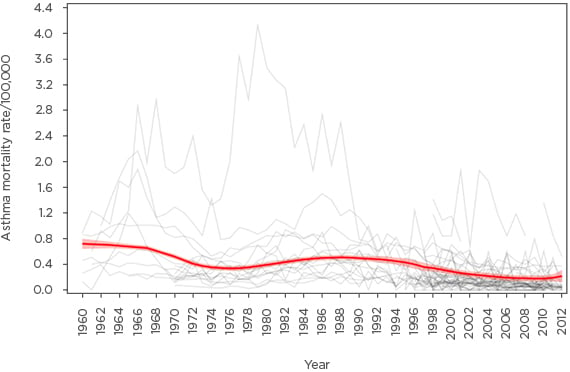
Figure 1: Crude asthma mortality rates from 1960–2012 for the 5–34 years age range in 46 countries and the two main eras of asthma management.
The locally weighted scatter plot smoother rates with 90% confidence intervals, weighted by country population, are shown in red. The association of the inflammation-based era with improved outcomes can readily be observed, as can the flat-line with regard to further improvements since 2005.
Adapted from Pavord et al.5
- Revolutionise airway disease and deliver precision medicine.
- Move beyond asthma control to prevention and cure.
- Emerge from age and discipline- associated silos.
- Test before treatment.
- Zero tolerance of asthma attacks.
- Maximise treatment opportunities in severe disease.
- Better research, especially basic and epidemiological.
The purpose of this article is to highlight some of the clinically important proposals of the Commission.
BEGONE, DULL ASTHMA!
The Commission aligns with previous suggestions that asthma is a term that has outlived its usefulness.9 Arthritis and anaemia were good umbrella terms for red, painful joints, and pale mucus membranes, respectively, but as clinically useful diagnostic terms they outgrew their usefulness decades ago and have been relegated from diagnosis to description. The same should be true of asthma; in the 21st century, it should be no more than a description of a symptom constellation (wheeze, breathlessness, chest tightness, and cough). The question ‘Is it asthma?’ should be replaced; instead, two questions should be asked: ‘Does the patient have an asthma?’ and, if so, ‘What sort of asthma does the patient have?’. The Commission builds on the insights of the late Dr Freddy Hargreave,10 who believed in deconstructing the airway, setting airway disease in the context of comorbidities and the environment (in the broadest terms), and especially in the concept of ‘treatable traits’.
As such, if the patient has an airway disease, the next question is: ‘What are the components of the airway disease?’ (Table 1). Once the airway disease phenotype has been determined, appropriate treatment can be planned. This approach is particularly useful in the context of wheeze in children of pre-school age, in the elderly, and also when deciding whether asthma is present in the context of other airway or systemic diseases. Thus, in pre-school children with a wheeze, rather than engaging in debates about whether you can diagnose asthma at an arbitrary age, the right approach is to delineate those children who have eosinophilic airway inflammation and are thus likely to benefit from a long-term commitment to ICS treatment. The recent INFANT trial,11 supported by other data,12 suggests that the presence of aeroallergen sensitisation and a raised peripheral blood eosinophil count (>300/μL) are predictive of a good response to ICS. In older children and adults, blood eosinophil count is a good predictor of response to mepolizumab13,14 and is more useful than previously used descriptive terms, such as asthma, chronic obstructive pulmonary disease (COPD), and asthma–COPD overlap syndrome.15 For patients with diseases like cystic fibrosis (CF), primary ciliary dyskinesia, and sickle cell anaemia, and for the survivors of premature delivery, rather than asking ‘Do they also have asthma?’, the questions that should be asked are: ‘Do they have eosinophilic airway inflammation and are they thus likely to benefit from ICS?’, ‘Do they have variable airflow obstruction and are they thus likely to benefit from short-acting β2 agonists?’, and ‘What are the treatable traits?’.
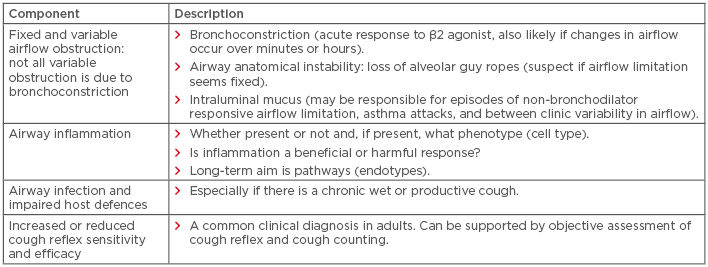
Table 1: Components of airway disease that need to be considered before deciding the nature of the asthma experienced by an individual.
Ultimately, we need to move from phenotypes (the set of observable characteristics of an individual resulting from the interaction of its genotype with the environment) to endotypes (a subtype of a condition, which is defined by a distinct functional or pathobiological mechanism). For example, one phenotype of airway disease is driven by persistent airway infection, characterised by neutrophilic inflammation, positive sputum culture, mucus hypersecretion, and secondary tissue damage. Phenotype-specific treatments are antibiotics, airway clearance, and mucolytics, all of which are nonspecific. CF is an endotype of chronic suppurative lung disease, caused by reduced or absent function of the CF transmembrane protein encoded by a gene on the long arm of chromosome 7. Understanding this endotype has resulted in moving from nonspecific, phenotype-driven therapies to an endotype-driven approach of pathway-targeted, specific therapies. An example of this is ivacaftor, a specific corrector of the molecular defect in Class III mutations that is increasingly being used in combination with other specific molecules to other gene classes. This should be the aim for asthmas, moving from airway eosinophilia to specific endotypes. Some progress has been made in this regard by the U-BIOPRED team,16 who have shown that there are at least two endotypes of airway eosinophilia: the first is enriched by gene signatures for IL-13/T helper cell type 2 and innate lymphoid cell type 2 cells, while the second is characterised by upregulation of metabolic pathway, ubiquitination, and mitochondrial function genes. This is still futuristic for most of the asthmas, and indeed it could be argued that it is irrelevant to most patients with eosinophilic asthma because they are easily treated with ICS. However, as discussed in the following section, endotypes have relevance beyond immediate treatment.
BEGONE, PALLIATIVE CARE FOR THE ASTHMAS!
Another recommendation from the Commission is that we need to move towards the prevention and cure of the asthmas, rather than just palliating the symptoms with inhalers; research to make a better preventative brown inhaler, or a longer acting blue inhaler, is of little relevance when we know standard therapy works for most asthmas if properly and regularly administered. There is abundant evidence that the asthma soil is prepared, and the seed is planted antenatally and throughout the pre-school years. It is exactly here that we need to urgently know the endotypes leading to progression to established eosinophilic asthma in particular. Without biomarkers of which patients will progress; and knowledge of the pathway down which they are progressing, our hopes of finding a cure or establishing primary or secondary prevention is negligible. We know ICS do not modify this pathway,17 so the initiating endotypes are likely to be different from those in established disease, and it is these early endotypes that hold the key to an asthma- free world.
The Commission has proposed a new approach to the evolution of asthma. There have been many birth cohort studies that have given us important insights into the evolution of asthma, although in many cases it is not immediately clear which asthma is being discussed. We now need to address the complexities of incorporating multiple small effects across the developmental trajectory into new models. New innovative approaches were proposed. Overall, the most important concept is that we must understand the progression of disease in order to stop it.
BEGONE, TALK OF ‘ASTHMA EXACERBATIONS’!
We have long railed against the word ‘exacerbation’ as a feeble description, implying a trivial and readily reversible event.18 In fact, the term ‘lung attacks’, which we prefer, carries an ominous prognosis in conditions as disparate as CF, primary ciliary dyskinesia, and interstitial lung disease. In the asthmas, lung attacks cause death, they are the single strongest predictor of further attacks and death, and they carry a longer-term risk of impaired airway development. We need to learn from the cardiologists and have a focussed response: why did the attack happen; what went wrong; was the asthma plan followed (if indeed it existed at all); does the plan need to be modified; was the patient adherent to treatment or were they over-using short-acting β2 agonists, under-using ICS, not attending regular check-ups, or having multiple emergency attendances. These are just a few of the issues that need to be considered. We should be aiming for asthma attacks to be seen as a catastrophic failure of management, not an issue that can be solved with a 3-day course of prednisolone.
The failure to recognise asthma attacks for what they are is inextricably linked to the trivialisation of the diagnosis and over-diagnosis of the condition. Letting inhalers become mere fashion accessories is of course expensive but has much worse consequences than being a waste of money. The lists of asthmatics in primary care become bloated by people with no or trivial airway disease, and the physicians despair of ever having time to do reviews of such huge numbers, and nothing happens. Would it be inappropriate to insist that having asthma can have consequences as serious as having cancer (i.e., dying)? As with cancer, the diagnosis should be objectively based and monitoring should be regular and taken seriously. An asthma attack is not dissimilar to the recurrence of cancer; it heralds a new and potentially fatal situation and requires a rapid response. An exaggerated view? Perhaps. But hopefully it provides a new view that offers correction to the affects of asthma on the modern world.
BEGONE, RESEARCH IMPRECISION!
Many aspects of improving research were discussed by the commissioners, but in this feature the problem of big asthma studies is discussed. The science of asthma research has never been more sophisticated, with genetic analyses and omics technologies integrated using novel systems biology. However, all too often the clinical inclusion criteria are superficial in the extreme, for example, ‘Doctor-diagnosed asthma’ or wheeze, despite the imprecision of this term. This is graphically illustrated by two genome-wide association studies. The Gabriel Consortium genotyped 10,365 patients with physician-diagnosed asthma and 16,110 unaffected people, and made very interesting discoveries, including the association of the ORMDL3/GSDMB locus on chromosome 17q21 with childhood onset asthma.19 A much smaller and more focussed study, including 1,173 cases and 2,522 controls aged 2–6 years, identified CDHR3 as an important gene.20 Cases were defined by recurrent severe attacks of wheeze requiring hospitalisation, and thus patients were truly likely to have one of the asthmas. The CDHR3 gene identification was an association missed by the much larger GABRIEL study.19,20 The importance of CDHR3 was further validated in vitro by the demonstration that the gene encodes for the receptor for rhinovirus-C.21 Clearly, we need to find better ways of phenotyping large numbers of patients for big-data studies; the CAMP study, for example, measured bronchial responsiveness as an entry criterion in >1,000 children.22 There is a clear need for biomarkers to diagnose and specify the type of asthma being studied for big genome-wide association studies and other studies if important findings are not to be missed.
CONCLUSION
The Lancet Commission poses significant challenges to the respiratory community. The core message is that asthma is an umbrella term, like anaemia and arthritis, describing a clinical syndrome while making no assumptions about underlying pathology. The next step is a greater commitment to making objective measurements to support a diagnosis, to deconstruct the airway and thus answer the question ‘What sort of asthma does the patient have?’, and to answer the same question in research subjects. We need to realise that we are badly letting down many people with an asthma. We propose a new age of personalised treatment of what should be appreciated as a killing disease. Above all, complacency needs to be swept aside and new thinking is needed if we are not to remain stuck in our present rut.







