Abstract
Osteoarthritis (OA) is the most prevalent form of arthritis worldwide and affects the whole joint. Changes in cartilage integrity, subchondral bone, and synovitis are recognised during OA progression. Although advances have been made in our understanding of OA pathophysiology, there are no current treatments that halt the progression of the disease. Treatments are largely based on physical therapies to improve joint function, anti-inflammatory agents to manage pain, and joint replacement surgery for late-stage disease in large weight-bearing joints. There is, therefore, an urgent need to better understand OA pathophysiology, which could help in the development of new treatments. The aim of this article is to review the evidence for structural correlates of pain and reduced joint function in OA; the data available for different joint compartments, including cartilage, bone, and the synovium, and their association with symptoms of OA are summarised and the use of imaging tools in assisting the understanding of OA pathophysiology is discussed. In recent years, more advanced imaging techniques, including MRI, have led to an improved understanding of changes at the bone–cartilage interface in OA, with a recognition that loss of integrity at this junction and development of bone marrow lesions (BML) in the subchondral bone are associated with OA pain in large epidemiological studies. One of the main challenges in OA BML research has been identifying the structural characteristics and patterns of gene and protein expression. Gene analyses of BML have demonstrated that they are highly metabolically active structures, providing evidence of angiogenesis, new bone and cartilage formation, and expression of neurotrophic factors. Findings from genomic and proteomic studies of BML, which are discussed in this review, have contributed to the identification of new molecular targets and an increase in our understanding of OA pathophysiology.
INTRODUCTION
Osteoarthritis (OA) is the most prevalent form of arthritis worldwide and is a leading cause of chronic pain and disability, resulting in a huge medical and socioeconomic burden.1,2 The condition most commonly affects ageing populations worldwide, compounded by the rise in common risk factors, including obesity, previous injury, and joint overuse.3 Current concepts consider OA to be a spectrum of overlapping disorders, often with a multifactorial aetiopathogenesis and common biological, morphologic, and clinical endpoints, resulting in the structural and functional failure of the synovial joint.4,5 OA can occur in all synovial joints but is most commonly seen in large weight-bearing joints, such as the hand, hip, knee, and spine, which have the greatest impact on disability.6
OA was previously considered to be a disease affecting articular cartilage; however, current theories suggest OA has a complex pathogenesis involving all structures of the joint, including the subchondral bone, ligaments, capsule, and synovial membrane.5,6 Visualisation of changes in the bone and other structures in the OA joint has been aided by the advent of MRI-based assessments.7 More recent studies have demonstrated that bone marrow lesions (BML) are an integral component of structural damage in OA and are likely to be a cause of pain.8 Large cohort studies, such as MOST,9 have supported observations that larger BML are associated with symptomatic OA and that synovitis, in particular detected by contrast-enhanced MRI, also contributes to OA symptoms of pain and impaired function. Other studies have also indicated increasing MRI-identified cartilage loss associated with OA progression; for example, in the large dataset of the OAI study.10
Joint degeneration often results in patients presenting with clinical symptoms, such as chronic pain, joint instability, and stiffness, leading these patients to seek care. As the condition worsens, structural changes become more apparent, including radiographic changes, such as joint space narrowing, osteophytosis, and subchondral sclerosis, often visible during disease progression. However, there is evidence to suggest a poor association between radiographic joint changes and symptoms, with pain sensitisation reported by many subjects in a study of knee OA.11 Recent work has also shown OA structural changes present in asymptomatic groups.12 There are data to suggest that certain stratified OA subsets can worsen over time, in comparison to other types of OA, for which symptoms can stabilise.12
There are currently few therapies that reverse OA progression or arrest its development. Recent studies have suggested that invasive ankle joint distraction may encourage cartilage regrowth and functional improvement.13 Furthermore, recently, knee joint distraction has shown evidence of tissue repair14 and knee osteotomy surgery has shown promise of cartilage regrowth and preservation of function.15 Nonsurgical management options include implementing nonpharmacological treatments to improve joint function and reduce stiffness, e.g., weight loss, low-impact exercise, and orthotics; and pharmacological management to relieve pain, e.g., analgesics, nonsteroidal anti-inflammatory drugs (NSAID), and intra-articular injections. In the late stages of OA, if severe pain persists and patients fail to respond to other recommended treatments, then surgery, e.g., joint replacement, is considered a recourse to improve quality of life, reduce pain, and improve joint function.16 The high unmet need to develop novel therapeutics for OA has led to the investigation of BML as a therapeutic target for OA, with recent studies showing improvement in knee OA pain, function, and BML size after zoledronate treatment.17 Inflammatory changes in the OA joint, e.g., synovitis, have also been the focus of treatment in a recent trial of hydroxychloroquine in hand OA;18 however, hydroxychloroquine was not found to be effective at improving pain or function in this type of OA.
IMAGING AND HISTOLOGICAL CHARACTERISATION OF BONE MARROW LESIONS
In recent years, the advancement of MRI techniques has improved methods of visualising bone and cartilage in OA, particularly at the bone–cartilage interface. Bone marrow signal changes were first described on MRI by Wilson et al.,19 who used the term ‘bone marrow oedema’ to describe MRI findings in painful joints. BML are observed frequently in people with OA and are often described as diffuse areas of high-intensity signal on T2-weighted, fat-saturated MRI or in short tau inversion recovery sequences.20 A large number of studies have delineated MRI BML changes in human subjects, particularly with large-joint OA.21-28 The results of a study of individuals with severe hip OA undergoing joint replacement established that the sum of BML measured via MRI corresponded to joint pain severity and the number of microfractures identified histologically.29 Kornaat et al.30 followed-up 182 OA subjects after 2 years and established that the total size of BML differed in 66% of the subjects, concluding that BML are involved in a dynamic process and, unlike cartilage loss, are not a static outcome. In addition, several longitudinal investigations have demonstrated that areas of BML are associated with subchondral cysts and could in fact be early precystic lesions.31 BML have also been strongly associated with cartilage degeneration, predominantly within areas overlying the lesion.5
With regard to histological analyses, studies have so far focussed on acquiring data from patients undergoing joint replacement surgery of the knee and hip. Zanetti et al.32 determined histologically that BML contain normal fatty marrow, with marrow necrosis, necrotic or remodelled trabeculae, oedema, and bone marrow bleeding. The same group matched MRI changes to BML abnormalities in a study of participants undergoing total knee replacement and found regions of normal tissue alongside bone marrow fibrosis, oedema, and bleeding.32 In a study of hip and knee OA, Hunter et al.33 reported increased bone volume fraction but decreased tissue mineral density within the BML using light microscopy. Samples from the lesion area showed increased trabecular thickness, with granulation, oedema, necrosis, fibrinoid deposition, and hyperplasia of the blood vessel walls. Taljanovic et al.29 reported one of the largest histological studies of hip OA BML to date, in which regions of fibrosis and microfracture formation at different stages of healing were observed. Leydet-Quilici et al.34 also described oedema, necrosis, and fibrosis within BML biopsies. Using MRI, Roemer et al. 35 previously demonstrated that progression of disease and the development of BML correlated with an increased risk of cartilage loss within the same subregion and that regions without BML were associated with a decreased risk of cartilage loss. Carrino et al.31 also reported that 87% of subchondral cysts were associated with BML abnormalities on MRI.
Several pathologies are seen histologically within BML, including bone remodelling, oedema, fibrosis, osteonecrosis, and trabecular abnormalities.32,36 Some theories propose that subchondral bone ischaemia may be an initiator for BML by impairing nutrient and oxygen exchange with articular cartilage, reducing cartilage integrity and increasing the risk of OA development.37-40 Others have proposed that bony micro contusions resulting in necrosis may lead to BML. Kijowski et al.41 suggested that increased intra-articular pressure leads to the permeation of synovial fluid and cellular infiltrate through defects in the articular cartilage into the subchondral bone, resulting in the proliferation of myxomatous tissue within the bone marrow.
PAIN AND BONE MARROW LESIONS
Recent work has shown that pain in OA arises from several structures within the arthritic joint, including the synovium (from which prostaglandins, leukotrienes, and inflammatory mediators are released), joint effusions, joint capsule involvement, and tendon and muscle weakness, which all contribute to reduced function.42 Synovitis is often observed by MRI in OA and strongly correlates with pain.35 Cartilage degradation is one of the hallmarks of OA disease43 and exposes the structures from which pain is most likely arising, since cartilage is an avascular, aneural structure composed largely of extracellular matrix (ECM) embedded sparsely with chondrocytes. There has been growing evidence of BML correlating with recurrent pain and pain severity in several studies, particularly in large-joint OA.44-46 A significant correlation was seen between the presence of larger BML in painful knees as opposed to non-painful knees.30,44-46 A study evaluating BML changes over time in patients with painful knee OA found that, within 3 months, BML scores decreased in 37 patients and increased in 71 patients.47 Epidemiological studies have shown a strong correlation between BML observed by MRI and OA-related knee pain in several large cohorts,44,46 with an odds ratio of 3.2 for the association of BML with pain. These data demonstrate the multifactorial nature of OA and how pain mechanisms are supported by the biopsychosocial model of pain.
GENE AND PROTEIN EXPRESSION IN BONE MARROW LESIONS
Although a large body of data now exists describing how BML are implicated in OA disease onset and progression, with descriptions of their histology and histomorphometry, very few studies evaluating the genetic and protein expression of BML have been conducted. This study group recently conducted a transcriptomic study of BML in OA.48 The novel findings demonstrated that BML have features of angiogenesis, fibrosis, new cartilage formation, and increased bone turnover, with disruption of the physiological osteochondral interface. In addition, a whole transcriptomic analysis of BML regions was used, finding upregulated expression of genes involved in neurogenesis, pain sensitisation, chemokine and cytokine signalling, as well as cartilage remodelling pathways.48 A multimodal approach with MRI to locate knee OA BML was also performed, followed by detailed histological analyses and whole transcriptomic techniques for a multivariate interrogation of the changes seen within BML. Detailed MRI matching with histological techniques allowed improved visualisation of BML, with direct observation of BML-associated cystic structures using MRI and transcriptomic expression analyses. Higher Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores with greater MRI Osteoarthritis Knee Score (MOAKS)-measured tricompartmental damage in advanced versus mild OA were found, as shown by previous studies.46
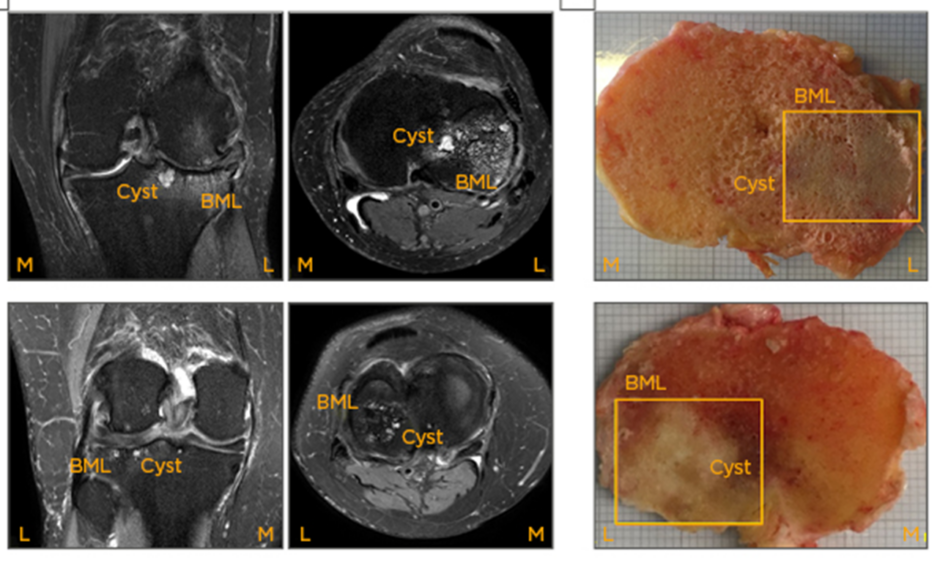
Cystic BML areas have been shown to be surrounded by regions of fibrosis, infiltration by inflammatory cells, and vascular proliferation. Previous hypotheses that BML could be precystic but that not all BML become cystic are also supported by the histological findings in this study and from other groups.31 It has been observed that cystic structures found within the BML areas using MRI were cysts but were also adjacent to areas of fibrocartilage, vascular proliferation, chondrogenesis, and amorphous tissue deposition (Figure 1). The authors also observed new cartilage forming deep within the subchondral bone compartment (Figure 1); this new cartilage tissue within the BML could be arising from mesenchymal stem cells (MSC) in the marrow, which has been seen by other groups.49 Campbell et al.50 reported altered numbers and phenotypes of MSC in cultures isolated from hip OA BML, showing that BML-derived MSC undergo osteochondral angiogenesis and have lower proliferation and mineralisation capacities in vitro.
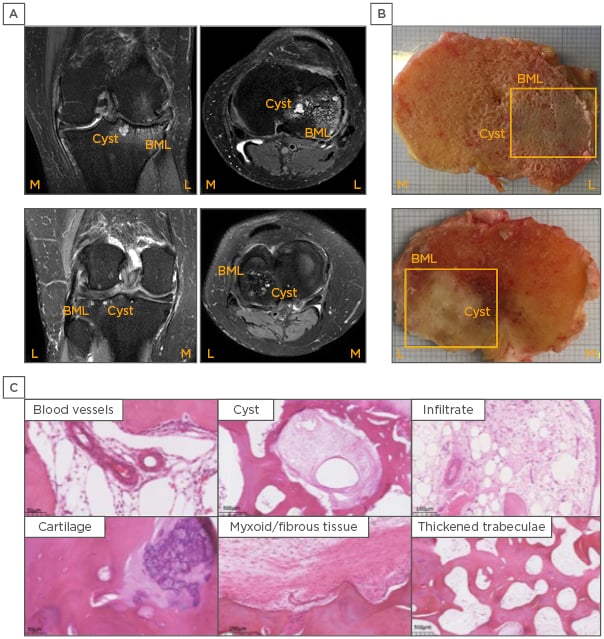
Figure 1: Imaging and histological analysis of a bone marrow lesion and the surrounding region.
A) Coronal and axial plane of a MRI scan visualising BML and an associated cyst. B) Macroscopic appearance of a BML identified by MRI at the time of tissue harvest during joint replacement surgery. C) Histology of a MRI-identified BML demonstrating typical pathological changes, including new blood vessels, cysts, cellular infiltrate, cartilage within the bone compartment, myxoid/fibrous tissue, and trabecular thickening.
BML: bone marrow lesion.
Microarray studies have shown that the highest upregulated gene from OA BML tissue was STMN2, encoding a phosphoprotein involved in regulating microtubule function, responsiveness to nerve growth factor, neuronal growth, and osteogenesis.48 Upregulation of STMN2 within BML may be involved in developing new neuronal structures and expansion of the BML in OA, thereby causing pain.51 However, STMN2 protein expression was higher in physiological bone than BML bone, which could reflect increased STMN2 turnover in OA BML or loss to the circulation due to increased tissue damage. The authors’ microarray study also identified neuronal markers, including THBS4, involved in the inflammatory response to central nervous system injury, presynaptic hypersensitivity, and neuropathic pain states.52 In animal models of pain sensitisation, THBS4 is increased locally in dorsal root ganglion neurons and contributes to pain behaviour, which can be inhibited by the calcium channel modulator gabapentin.53
Genes implicated in neuronal morphogenesis have also been identified in BML, including ATP6V0D2, PSIP1, NYAP2, FERM, and FRMPD4, encoding proteins implicated in central nervous system development and pain states.54,55 ECM genes have also been associated with BML, including MMP13 and collagen genes, COL16A1, and those encoding fibronectins and growth factors that are known to be bound within the ECM.56
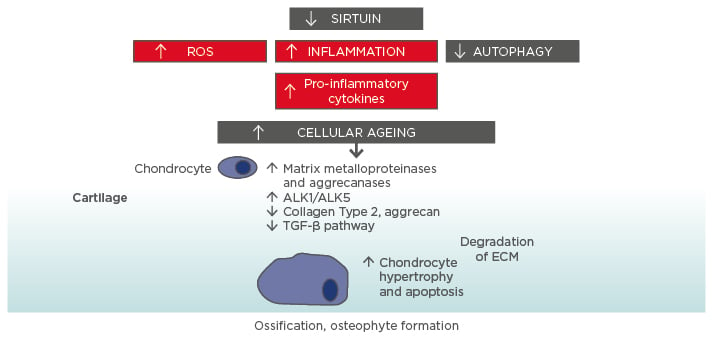
BML are regions of high metabolic activity with increased cell turnover, bone remodelling, and neuronal and inflammatory gene signatures, and gene ontological analyses have revealed the involvement of canonical pathways in chemokine, integrin, and cytokine signalling. There is evidence of neurodevelopment and pain pathway signalling represented by the Alzheimer’s, Notch, catenin, and Wnt pathways, alongside vascular endothelial growth factor and angiogenic pathway expression. Studies by Hopwood57 and Chou et al.58 analysing the gene expression profiles of OA bone showed expression of bone remodelling signalling pathways, including Wnt, TGF-β, and bone morphogenic protein (BMP), and bone remodelling molecules, such as periostin and leptin. Additionally, Kusumbe et al.59 described how the growth of blood vessels in bone and osteogenesis are coupled, proposing that Type H endothelial cells mediate local growth of the vasculature and provide specific signals for perivascular osteoprogenitors. The same group reported that endothelial Notch activity promotes angiogenesis and osteogenesis in bone.60 The authors have also demonstrated OMD expression in BML tissue and Ninomiya et al.61 showed that osteoclast activity induces OMD expression in bone, suggesting BML represent areas of active bone remodelling.
The expression of angiogenic and osteogenic genes, along with the tissue changes identified in BML, suggest that vascular proliferation and bone formation are likely to be coupled in BML development. Since blood vessels are formed within neurovascular bundles, it is likely that the increased neuronal pathway gene expression, including STMN2, THBS4, PSIP1, NYAP2, and genes encoding catenin, which are among some of the most highly expressed genes in BML analyses, are implicated in neural pathway development, nerve formation, and pain mediation in BML tissue.
Gene arrays of BML have also identified molecules within the Wnt signalling pathway, including catenin, and other studies have demonstrated a critical role of Wnt signalling in the production and persistence of neuropathic pain after nerve injury and bone cancer.62 Studies using rodent models have suggested that in nerve injury and bone cancer pain models, Wnt signalling is activated, which may contribute to pain by regulating proinflammatory cytokines IL-18 and TNF-α, respectively, as well as NR2B and subsequent Ca2+-dependent signals in the dorsal horn. This analysis also showed a high representation of inflammatory chemokines and cytokine signalling, while other groups have also identified chemokines involved in OA pain; for example, CCR2 was recently reported to mediate pain in a murine model of OA.63 Microarray data suggest that chemokine pathway molecules could be pain sensitisers in BML.48 Walsh et al.64 highlighted that OA neurovascular changes at the osteochondral junction, including vessels and both sensory and sympathetic nerves breaching the tidemark, could be a source of joint pain. The genes identified in BML transcriptomic analyses support the hypothesis of neurovascular gene upregulation in BML tissue.
One of the most highly expressed genes in BML tissue is MMP13, traditionally thought to encode an enzyme expressed in cartilage that is involved in regulating ECM turnover and cartilage destruction in OA.65 Increased bone matrix microdamage and altered vasculature in the subchondral bone of BML have also been demonstrated in a recent histological study of 73 subjects undergoing knee joint replacement surgery.66 Supporting the importance of matrix turnover in OA, data have shown that Type II collagen degradation products are increased in the urine of subjects with advanced OA who had MRI-identified BML.48 The de novo cartilage formation observed within BML, coupled with the increased transcriptomic expression of MMP13 observed using microarray and the detection of MMP13 cleavage products, could suggest recapitulation of the embryonic bone development phenotype within OA BML regions.
Although much has been learned over the last few years with respect to gene expression and protein turnover at the osteochondral interface in OA, further validation of the molecules identified and their relative importance in distinct stages of OA pathophysiology is required. Validation of genes and proteins involved in matrix turnover, angiogenesis, and neurogenesis will not only assist in OA disease stratification but will also identify therapeutic targets.67 It is conceivable that information on pertinent molecular markers in OA, coupled with MRI data, may assist in improved patient stratification that could help in developing more targeted treatments for people with OA.
SUMMARY
Recent work, supported by MRI studies and tissue analyses, has demonstrated that OA is a disease of the whole joint. Symptoms of pain and impaired function are influenced by joint synovitis, joint effusion, tendon and ligament involvement, and the presence of BML, which are most easily detected by MRI. BML in OA represent regions of high metabolic activity, with expression of genes involved in neuronal development, pain, ECM turnover, cartilage and bone formation, and angiogenesis. Recent work focussing on the bone–cartilage interface in OA and the deeper underlying bone has revealed that BML development is an important step in OA pathogenesis. The new findings from gene array and protein studies of BML suggest the possibility of developing new diagnostic tools; for example, identifying angiogenesis and bone turnover markers based on ECM turnover. However, further evaluation is required of the genes associated with specific markers of BML region, size, and severity. It is also possible that the enlargement of BML is associated with specific markers identified from gene array studies, which requires further work to evaluate BML at different stages of development and formation. It is conceivable that in the future, combined testing of molecular markers identified from the synovia, cartilage, and BML with MRI of the target joint may be used to assess the degree and severity of damage of an OA joint. The findings from BML functional tissue studies also raise the possibility of developing future therapies targeting BML formation and turnover for OA, which is the most common arthritic disease worldwide.