Abstract
Heart failure (HF) with a preserved ejection fraction (HFpEF) is now the predominant HF subtype in Europe. It manifests as reduced cardiac output and/or increased left ventricular filling pressures at rest and/or during exercise, caused by left ventricular diastolic dysfunction. HFpEF is proposed to occur as a result of systemic microvascular endothelial inflammation associated with various comorbidities such as hypertension, obesity, and diabetes. However, nearly two-thirds of those with HFpEF are females, which points to sex-specific driving factors such as differences in cardiac structure and physiology, and in systemic and pulmonary circulation; oestrogenic influence on physiology and molecular mechanisms; and pregnancy-related hypertensive disorders. Pharmacotherapy for HFpEF is lagging behind that for HF with reduced ejection fraction, and no treatment has yet been convincingly shown to reduce morbidity or mortality. Current treatment strategies target symptom alleviation and comorbidities. No trials have specifically examined the difference between sexes in response to HFpEF treatment; however, post hoc analyses have revealed differing effects of some treatments according to sex, such as spironolactone or sacubitril/valsartan, which may be of more use in females with HFpEF than in males. Further studies are needed to confirm if better outcomes were because of specific female physiology and HFpEF pathophysiology, and whether the outcome measures can be more tailored to address the goals of female participants. This paper highlights some key differences between females and males with HFpEF, discusses clinical trials assessing treatments, and proposes what is needed to target HFpEF care according to sex.
INTRODUCTION
Heart failure (HF) represents a major public health issue, with prevalence approaching 2% in most European countries and considerable associated morbidity, mortality, and healthcare expenditure.1 The European Society of Cardiology (ESC) proposed a classification of HF subtypes according to left ventricular ejection fraction (LVEF): ‘HF with preserved ejection fraction’ (HFpEF) when ≥50%, ‘HF with reduced ejection fraction’ (HFrEF) when <40%, and ‘HF with mid-range ejection fraction’ when between 40% and 50%.2 While HF symptoms and/or signs with reduced LVEF define HFrEF, diagnostic criteria for HFpEF/HF with mid-range ejection fraction are more complex, requiring elevated natriuretic peptide levels and at least one additional criterion of structural heart disease and/or left ventricular (LV) diastolic dysfunction.2 Two more refined diagnostic approaches of HFpEF using scores that take into account multiple clinical, biological, echocardiographic, or invasive haemodynamic parameters have been proposed, but still need confirmation in clinical practice before being adopted.3,4
In the last 20 years, the proportion of people with HFpEF has increased relative to those with HFrEF, resulting in HFpEF being the predominant HF subtype in Western countries, representing >50% of those with HF.1,5 In Europe, an estimated 4.9% of the general population aged ≥60 years have HFpEF.6 Demographic and clinical characteristics of people with HFpEF clearly differ from those with HFrEF. For instance, the former are on average 6 years older and approximately 63% are females, as opposed to 43% with HFrEF.5 In addition, several cardiovascular (CV) and non-CV comorbidities, such as hypertension, chronic kidney disease, atrial fibrillation, and diabetes, are more frequent in HFpEF,1,5 and play a role in its pathogenesis.1 Regarding clinical outcomes, while CV mortality is lower, non-CV mortality is slightly higher in people with HFpEF compared to HFrEF.5 However, several studies have reported similar outcomes regarding hospitalisation rates and quality of life (QoL) regardless of HF diagnosis.7,8
Until now, no treatment has proven effective in reducing morbidity and mortality in HFpEF. This is particularly relevant for females, who are over-represented in this HF category. A recent HFpEF trial indicated that females may have different responses to therapy compared with males;9,10 therefore, a sex-specific approach to HFpEF assessment and treatment could result in improved treatment efficacy. This review explores sex differences with regard to HFpEF pathophysiology, outcomes, and pharmacotherapy. Potential underlying mechanisms and clinical implications are also discussed.
PATHOPHYSIOLOGY OF HEART FAILURE WITH A PRESERVED EJECTION FRACTION AND RELATIONSHIP WITH SEX
The central pathophysiological alteration in HFpEF is LV diastolic dysfunction (impaired relaxation and increased diastolic stiffness) leading to abnormal haemodynamics, namely reduced cardiac output and/or increased LV filling pressures at rest and/or during exercise. HFpEF was previously termed ‘diastolic HF’, but this nomenclature was abandoned as a certain degree of LV diastolic and systolic dysfunction coexists in all HF subtypes and nondiastolic and noncardiac mechanisms are involved in HFpEF. Traditionally, LV diastolic dysfunction is associated with a concentric pattern of LV remodelling, characterised by normal end-diastolic LV volume and increased LV wall thickness, frequently secondary to systemic arterial hypertension.11 However, many with HFpEF exhibit normal chamber structure without remodelling.
A pathophysiological concept is proposed in which comorbid conditions, such as hypertension, overweight/obesity, diabetes, chronic obstructive pulmonary disease, sedentary lifestyle, or iron deficiency, create a systemic microvascular endothelial inflammation.
These lead to microscopic myocardial inflammation and fibrosis, increased oxidative stress, and alterations in cardiomyocyte signalling pathways, causing cardiomyocyte remodelling and dysfunction.12 Myocardial blood flow is reduced in HFpEF patients without obstructive coronary disease, further emphasising the role of the microcirculation in HFpEF development.13 Furthermore, there are important roles for subtle abnormalities in systolic function,14-16 arrhythmias (particularly atrial fibrillation),16,17 pulmonary vascular and right ventricular function,19,20 stiffness and dysfunction of large and small arteries,21-23 and skeletal muscle.24,25
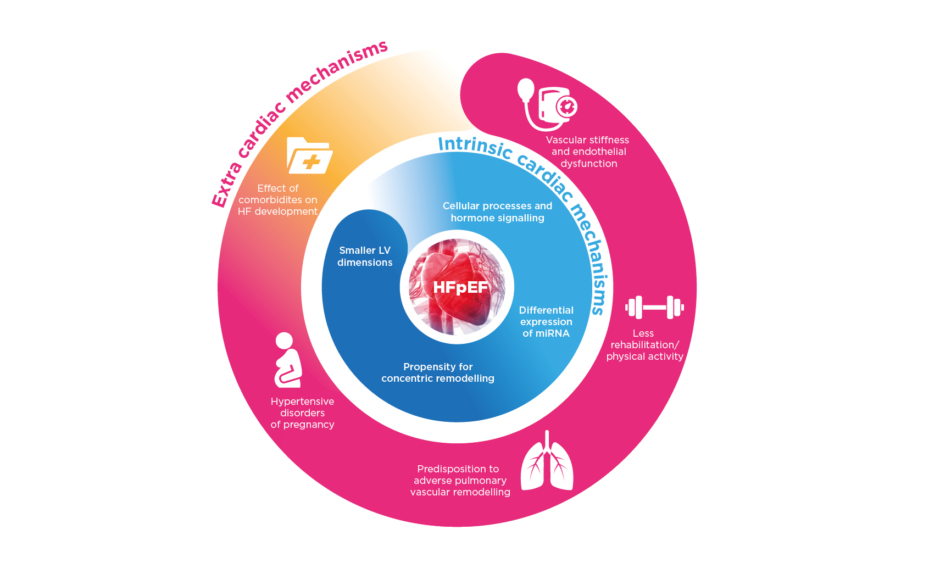
Females are predisposed to develop HFpEF and exhibit differences in disease phenotype. Female sex is associated with 2.8-times greater odds of having HFpEF compared with HFrEF.26 Sex differences in HFpEF pathophysiology include structural and functional cardiac/noncardiac factors and comorbidities (Figure 1).27
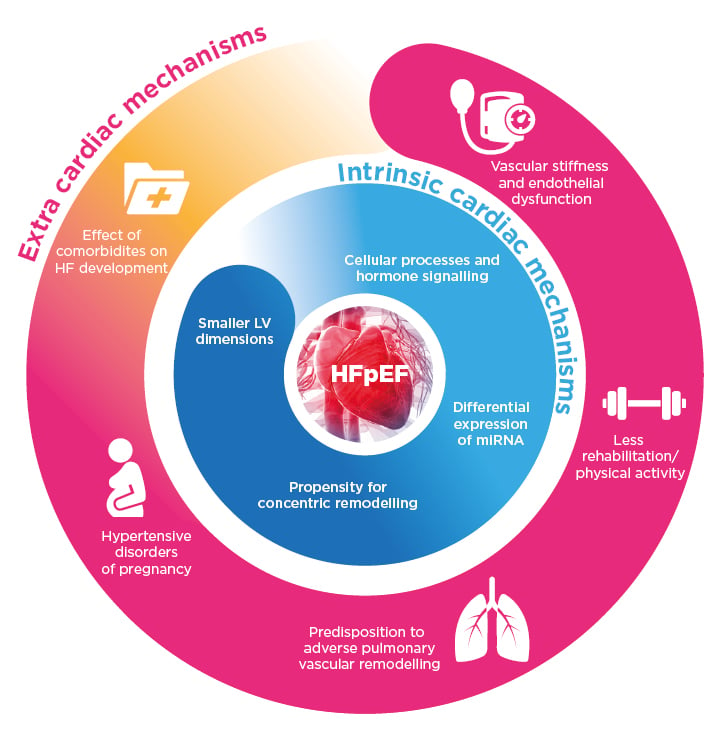
Figure 1: Potential mechanisms behind the pathophysiology of heart failure with a preserved ejection fraction in females.
HF: heart failure; HFpEF: heart failure with preserved ejection fraction; LV: left ventricular; miRNA: micro RNA.
Compared with males, females have smaller LV dimensions and lower stroke volumes, even accounting for body size; as such, heart rate needs to more greatly increase during exercise in females, which is exacerbated with ageing.27 Females are also more prone to increased LV wall thickness, concentric remodelling, and diastolic dysfunction, particularly when accompanied by hypertension.28,29 Additionally, LVEF is on average higher and increases more with age in females, though they develop a greater reduction in systolic long-axis contraction velocity.30,31 Also with increasing age, males tend to lose myocardium and LV chamber size increases whereas for females, LV mass and chamber size can remain fairly stable, rendering females more at risk from chronotropic incompetence and systolic/diastolic impairment seen in HFpEF, particularly on exertion.27,32
The underlying molecular and cellular mechanisms predisposing females to LV diastolic dysfunction remain largely unexplained, though many different, primarily hormonally-mediated, hypotheses have been proposed.27 For instance, oestrogen is involved in downregulation of protein kinase A, which modulates phosphorylation of the sarcomeric structural protein titin/connectin involved in cardiomyocyte stiffness.33 Somewhat greater LV concentric hypertrophy is also associated with oestrogen, attributed to growth factor modulation.34 Oestrogen levels also control transcription and processing of some microRNA, which affect post-transcriptional cellular processes, with several microRNA known to escape X-chromosome inactivation more expressed in particular female cell types. These microRNA can notably influence response to metabolic stress and vascular inflammation of endothelial cells, vascular smooth muscle cells, and cardiomyocytes. They may promote endothelial dysfunction, smooth muscle cell proliferation, and cardiac hypertrophy, among other mechanisms involved in HFpEF.35
Sex differences in systemic and pulmonary circulation may play a role in HFpEF development. For instance, increased vascular stiffness and endothelial dysfunction predominantly affect females.32,36 Depending on artery size and localisation, pathophysiological consequences include increased wave reflection with greater increase of ventricular elastance, abnormal ventricular–arterial coupling, and coronary microvascular dysfunction.27 Pulmonary vascular reactivity and remodelling can vary between sexes. For example, females are more affected by idiopathic pulmonary arterial hypertension.37 In HFpEF studies, many more females are noted in cohorts with pulmonary hypertension (82%) compared to those without (58%).38 Lastly, HFpEF comorbidities are also important, particularly because of their contribution to systemic inflammation. Many are more prevalent in females, including iron deficiency, obesity, or autoimmune disease; more frequently associated with HF, such as hypertension and diabetes; or present uniquely, as in pregnancy-related hypertensive disorders.27
Several studies have highlighted sex differences in HFpEF phenotype. The largest echocardiographic study found females with HFpEF were more likely to have concentric LV remodelling with smaller LV diameters and higher LVEF.39 In one haemodynamics study (n=161), compared to males, females with HFpEF had a higher pulmonary capillary wedge pressure (PCWP) indexed to peak exercise workload and a smaller rise in stroke volume index with exercise, indicating poorer diastolic reserve. Systemic and pulmonary compliance levels were also lower in females at rest and during exercise.36 In another study, adding cardiopulmonary exercise testing during right heart catheterisation with repeated measures of PCWP and cardiac output found a steeper PCWP/cardiac output slope during exercise in females, indicating greater diastolic reserve impairment.40 In addition, females exhibited poorer peripheral oxygen extraction and worse right ventricular and LV systolic reserve during exercise.
IS THERE A DIFFERENCE IN RESPONSE TO THERAPY BETWEEN MALES AND FEMALES?
Therapies attempted in HFpEF generally mirror those with efficacy in HFrEF, such as angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, angiotensin receptor neprilysin inhibitors, mineralocorticoid receptor antagonists, and β-blockers. However, no treatment has yet been convincingly shown to reduce morbidity/mortality associated with HFpEF.2,9 Current therapeutic strategies in HFpEF comprise first alleviating congestion with diuretics, then treating comorbidities. A combined endurance/resistance rehabilitation training programme should also be proposed to improve exercise capacity and QoL.2
No trials have specifically examined sex differences in response to HFpEF treatments; however, post hoc analyses of five HFpEF trials have reported such differences in baseline characteristics and clinical outcomes or in response to therapy (Table 1).10,41-44
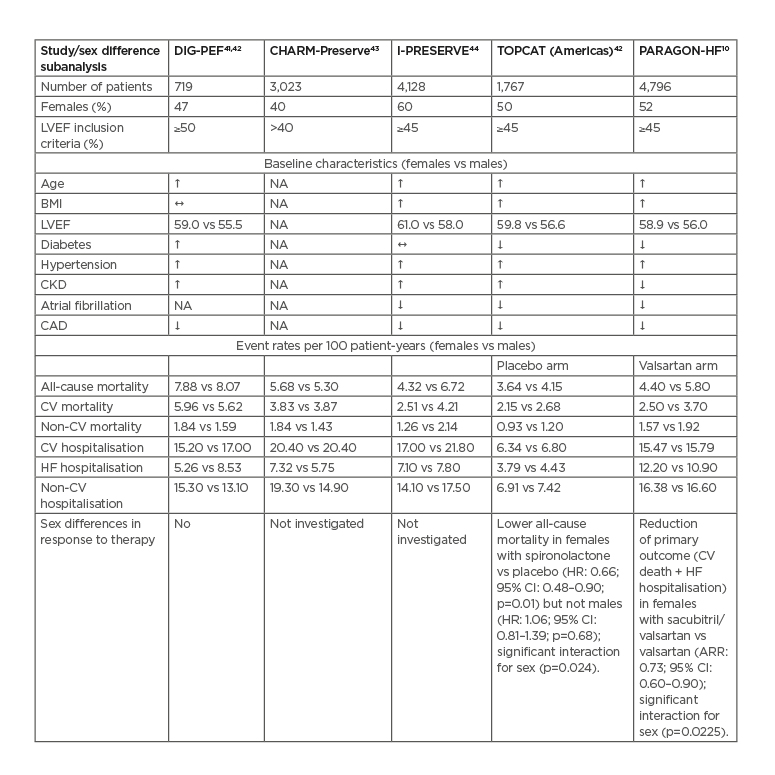
Table 1: Sex-related differences in trials of heart failure with a preserved ejection fraction.
#: statistically significantly higher in females
$: statistically significantly lower in females
1 : no statistically significant difference
ARR: Adjusted rate ratio; CAD: coronary artery disease; CI: confidence interval; CKD: chronic kidney disease; CV: cardiovascular; HF: heart failure; HR: hazard ratio; LVEF: left ventricular ejection fraction; NA: not analysed.
In the primary DIG trial45,46 (LVEF >45%; n=988), digoxin use was not associated with any effect on mortality or hospitalisations with HFpEF and no significant heterogeneity in treatment effect between sexes were reported. However, subanalysis of baseline characteristics of 719 patients with a LVEF ≥50% (n=341 females)41 did reveal differences prior to therapy. Females were on average 3 years older, had higher blood pressure (BP) and LVEF, and had more symptoms and signs of advanced HF, but less coronary artery disease (CAD) and chronic kidney disease. After adjustment for baseline differences, female sex was an independent predictor of lower mortality (hazard ratio [HR]: 0.59; 95% confidence interval [CI]: 0.43–0.82), although HF hospitalisation rates were similar between sexes (HR: 1.06; 95% CI: 0.75–1.51).41
Another secondary analysis confirmed better survival in females compared to males for both HFrEF and HFpEF. Hospitalisation rates were similar for HFpEF, but greater in males with HFrEF.47
Candesartan versus placebo was investigated in the CHARM-Preserved programme (LVEF >40%; n=3,023; 40% females).48 In general, candesartan had neutral effects on mortality but a modest reduction in HF hospitalisations. Further analysis found females had better outcomes for mortality and HF hospitalisations compared with males, regardless of baseline LVEF.42 More specifically, adjusted HR for all-cause mortality in females versus males was 0.77 (95% CI: 0.69–0.86; p<0.001) and 0.83 (95% CI: 0.76–0.91; p<0.001) for CV death or HF hospitalisation.43
The I-PRESERVE trial49 compared irbesartan to placebo (LVEF ≥45%; n=4,128; 60% females; ≥60 years). Irbesartan did not improve the primary outcome (death from any cause or hospitalisation for a CV cause) or other prespecified outcomes, and no sex differences were observed. A subanalysis found that at baseline, females were on average 1 year older and more likely to be obese and have chronic kidney disease and hypertension, but were less likely to have ischaemic heart disease, atrial fibrillation, or chronic obstructive pulmonary disease.43 During a mean follow-up of 49.5 months, the primary outcome unadjusted HR was 25% lower in females than males (HR: 0.75; 95% CI: 0.68–0.83; p<0.001) and remained significant after adjusting for baseline covariates (adjusted HR: 0.81; 95% CI: 0.72–0.92; p=0.001). Four baseline characteristics seemed to particularly mitigate this finding: atrial fibrillation, renal dysfunction, stable angina pectoris, or advanced New York Heart Association (NYHA) Class symptoms.43
In the TOPCAT trial44 (LVEF ≥45%; n=3,445; 52% females),50 spironolactone did not significantly reduce incidence of the primary composite outcome (CV death, aborted cardiac arrest, or HF hospitalisation) compared to placebo. Subgroup analysis did not show heterogeneity in treatment effects according to sex. However, in a secondary analysis, sex differences in outcomes and responses to spironolactone in 1,767 patients (50% females) found females were older and had higher LVEF, BP, and BMI, with fewer comorbidities.44 There were no significant differences in outcomes between sexes in the placebo group. Spironolactone use was associated with reduction in all-cause mortality in females but not in males, with a significant sex-treatment interaction (Table 1).44 These findings indicate a possible sex-specific benefit of spironolactone in females, but require confirmation.
Using individual patient data from the CHARM-Preserved, I-PRESERVE, and TOPCAT trials, a meta-analysis (4,458 females, 4,010 males; LVEF ≥45%) found that females were older and more were classed as obese and hypertensive, but were less likely to have CAD or atrial fibrillation.51 Primary outcome risk (composite of HF hospitalisation or CV death) was lower in females compared with males (HR: 0.80; 95% CI: 0.73–0.88), with a similar risk of HF hospitalisation but a lower risk of sudden cardiac death.51
Finally, the PARAGON-HF trial9 compared the angiotensin receptor neprilysin inhibitor sacubitril/valsartan to valsartan alone (n=4,822; mean age: 73 years; 52% female; mean LVEF: 58%). Primary outcome occurrence rate (composite of total hospitalisations for HF and death from CV causes) was slightly, but not significantly, reduced in the sacubitril/valsartan group versus the valsartan group (rate ratio: 0.87; 95% CI: 0.75–1.01; p=0.06). Prespecified subgroup analysis of the primary outcome indicated heterogeneity of treatment, with possible benefits of sacubitril/valsartan in patients with lower LVEF (≤57%) and in females. Dedicated analysis found that compared with males, females in the PARAGON-HF trial were on average 2 years older, had higher LVEF, lower N-terminal pro B-type natriuretic peptide (NT-proBNP), less CAD, and less atrial fibrillation. Compared to valsartan, the unadjusted rate ratio for the primary outcome with sacubitril/valsartan versus placebo was 0.73 (95% CI: 0.59–0.90) in females and 1.03 (95% CI: 0.84–1.25) in males (p interaction: 0.017), results that persisted after adjustment for sex differences in baseline covariates and that were exclusively driven by a reduction in HF hospitalisation (Table 1).10
POTENTIAL UNDERLYING MECHANISMS OF SEX DIFFERENCES IN CLINICAL OUTCOMES AND RESPONSE TO THERAPY
It is largely unknown why females with HFpEF have better mortality outcomes even after adjustment for potential confounders, though several hypotheses have been proposed. Most importantly, females in all trials were invariably less affected by CAD and therefore at lower risk for sudden cardiac death, the main driver of mortality in the pooled clinical trial cohort.51 Even if analyses were adjusted for CAD, some, predominantly males, with silent disease may not have been diagnosed.
The sex differences in response to spironolactone and sacubitril/valsartan observed in the secondary analyses of TOPCAT and PARAGON-HF may be due to chance, but several other mechanisms may be postulated (Figure 2).
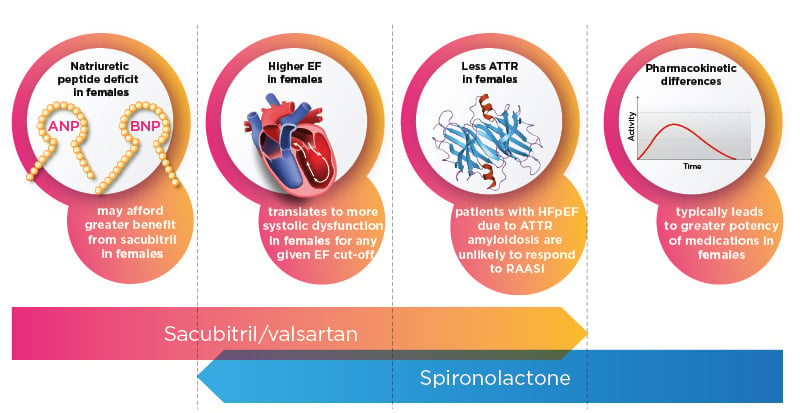
Figure 2: Possible mechanisms behind sex differences in response to sacubitril/valsartan and spironolactone.
ANP: atrial natriuretic peptide; ATTR: transthyretin amyloidosis; BNP: B-type natriuretic peptide; EF: ejection fraction; HFpEF: heart failure with preserved ejection fraction; RAASi: renin–angiotensin–aldosterone system inhibitors.
Females with HFpEF may be better responders because their HFpEF pathophysiology is different. As previously mentioned, females in general, and specifically with HFpEF, have on average higher LVEF than males. This implies that in females even a preserved LVEF of 55% may be associated with mild alterations of other indices of systolic function, which were not considered in the above-mentioned analyses. As both spironolactone and sacubitril/valsartan have clear beneficial effects in HFrEF, they may also be more effective in females with HF and higher ranges of LVEF compared with males.10
A greater biological activity of spironolactone in females may be another explanation. In the active group of TOPCAT, greater increases in creatinine and potassium were observed, without any significant difference in the remaining medications. This indicates a more potent renal effect of spironolactone in females compared with males.44 Animal studies have also shown a greater impact of mineralocorticoid receptor antagonists on cardiac remodelling in females versus males.51 It could be that there is a greater myocardial profibrotic effect of aldosterone in females, though this has not yet been demonstrated. In PARAGON-HF, while there was no biological signal indicating a greater effect of sacubitril/valsartan in females as decreases in BP or NT-proBNP were similar in both sexes, other signals may not have been detected. Recent data from the BIOSTAT-CHF registry in HFrEF indicated that females achieve the lowest risk of death or hospitalisation from HF pharmacotherapy at 50% of the recommended doses with no further benefits at higher doses, whereas males have the best outcomes at 100% of the recommended dose.52
In TOPCAT and PARAGON-HF, it may be postulated that spironolactone was more effective because it was used at a relatively higher dosage for females compared to males, even if the absolute dose was not different.
In PARAGON-HF, natriuretic peptide levels were lower in females despite more advanced HF signs and symptoms, partly because they had more visceral obesity compared with males. This natriuretic peptide deficit in females may explain why they benefit more from sacubitril/valsartan which, by the action of sacubitrilat, the active metabolite of sacubitril, inhibits neprilysin and thus increases natriuretic peptides. Neprilysin is an endopeptidase that degrades dozens of different vasoactive peptides. Females may have a different panel of these peptides and therefore a different response to sacubitril/valsartan. For instance, females are more prone to develop angioedema with neprilysin inhibition, which is mediated by bradykinin.10
Finally, wild-type transthyretin cardiac amyloidosis is increasingly shown as an important cause of HFpEF and may affect 13% of those hospitalised for this category of HF.53 As there is a clear male predisposition to this condition (>90% of cases),54 a significant proportion of males may have been affected in TOPCAT and PARAGON-HF by unrecognised wild-type transthyretin cardiac amyloidosis, which is known not to respond to renin–angiotensin–aldosterone inhibition and to have a poor prognosis.53,54
AREAS FOR FURTHER EXPLORATION AND FUTURE DIRECTIONS
Theoretically, sex differences observed in subgroup analyses of the TOPCAT and PARAGON-HF trials are hypothesis-generating only and should be confirmed in new trials conducted specifically in females. There is also significant scope to further explore underlying pathophysiologic and phenotypic differences in HFpEF between the sexes, particularly given that the heterogeneous nature of HFpEF has been postulated to be a significant contributor to the neutral outcome of multiple HFpEF trials.55 Trials targeting underlying mechanisms behind HFpEF that are particularly problematic in females, such as pulmonary vascular dysfunction, greater pulsatile afterload and greater LV filling pressures, and lower stroke volume recruitment with exercise,56 may be more effective than the application of existing treatments for HFrEF, which less commonly affects females.
Current trials of new therapies for HFpEF are underway that may address underlying mechanisms of HFpEF that are particularly relevant to females. First of all, the transcatheter InterAtrial Shunt Device (Corvia Medical, Inc., Tewksbury, Massachusetts, USA) has shown promising results in the REDUCE LAP-HF trial by reducing PCWP with exercise,57 which could be of greater utility in females. Pulmonary vascular dysfunction is a key component of exercise intolerance in females with HFpEF.36 As such, a trial investigating a specific pulmonary vasodilator, macitentan, in people with HFpEF and pulmonary vascular disease is underway (SERENADE).58 Finally, two other HFrEF therapeutic strategies currently being tested in HFpEF, sodium glucose cotransporter inhibitors (EMPEROR-PRESERVED;59 DELIVER60) and oral soluble guanylate cyclase activators (Vitality-HFpEF),61 could be of particular interest to females in whom diabetes and arterial stiffness play an important role in HFpEF.
Another important consideration is the outcome measure used in trials of HFpEF, particularly in females. The marked sex difference in mortality outcomes described above highlights that studies with a CV mortality primary endpoint are of less relevance to the female HFpEF population. Rather, a focus on improving symptoms and QoL, along with hospitalisations, may be more meaningful as females with HF consistently report lower QoL and greater rates of anxiety and depression.7 Treatments with a greater focus on symptomatic improvement and exercise tolerance warrant further exploration and may involve great investment in nonpharmacological therapeutic options, such as exercise regimens and weight loss, particularly given that this is underutilised among females.62
Finally, sex differences in key risk factors that predispose to HFpEF development require attention. For example, aggressively targeting systemic hypertension in females is a key component to HFpEF prevention; however, despite a greater prevalence of hypertension than males, particularly after menopause,63 females have a lower rate of control when treated pharmacologically.64 The development of hypertension following menopause is driven by the reduction in oestrogen causing both increased vascular resistance through a reduction in vasodilatory and anti-inflammatory pathways and a rise in sympathetic nervous system activity.65 While hormone replacement therapy has proven ineffective in CV disease prevention in large studies, targeted oestrogen modulation could be investigated, particularly for the treatment of hypertension and HFpEF in women. Sex-specific preventative strategies for HFpEF should also address obesity, which affects more females than males globally.27 Similarly, there are a number of risk factors specific to females (e.g., hypertensive disorders of pregnancy) or that disproportionately affect females (e.g., autoimmune disorders) that may play a significant role in HFpEF development and for which there is very little framework at present to minimise future CV risk.27
CONCLUSION
In the context of increasingly recognised sex dimorphisms in the pathophysiology and phenotypes of HFpEF, it is unsurprising that responses to therapy differ. Although trials of pharmacologic therapies for HFpEF have been neutral, they have highlighted important differences in outcomes between the sexes, along with some signals for sex differences in the response to spironolactone and sacubitril/valsartan for HFpEF. This requires further exploration with dedicated trials, along with studies investigating therapies targeting the key pathophysiologic pathways and phenotypic features seen in females. Furthermore, trials of therapies for HF in females with broader outcomes encompassing QoL, rather than focussing on mortality, may be more relevant to the female population.