Meeting Summary
The objectives of the symposium were to raise awareness of the importance of treating early, setting treatment goals, and using enhanced clinical monitoring in inflammatory bowel disease (IBD). The progressive nature of Crohn’s disease (CD) leading to bowel damage is well-established, but, according to Prof Peyrin-Biroulet, there may be a window of opportunity early in the disease when progression can be prevented through early diagnosis coupled with early intervention. The same approach should be adopted for the treatment of ulcerative colitis (UC), which he noted is frequently undertreated. UC is also progressive and the overall disability associated with UC is similar to CD.
Prof Colombel described the treat to target (T2T), with tight control (TC), approach in IBD. The target is a composite endpoint of clinical and endoscopic remission, determined and agreed upon with the patient. In this approach, the disease is continuously monitored and treatment modified until the target is reached with the primary aim of blocking disease progression. The CALM study1 demonstrated that a significantly higher proportion of patients in the TC arm achieved mucosal healing at 1 year compared to patients with a conventional treatment management. In order to illustrate the benefits of early diagnosis, Prof Panaccione presented two cases from clinical practice who exhibited similar symptoms at disease onset. The first case took 3 years to present; her treatment was managed conventionally and escalated according to symptoms with no assessment of biomarkers. She had recurrent symptoms and eventually required ileocaecal resection. By contrast, in the second case, diagnosis occurred within 4 months of symptom onset, and biomarkers were assessed. Biological treatment was initiated at the second consultation and optimised with a TC approach. The treatments in both cases were similar; however, conventional management resulted in disease progression and the T2T approach with TC resulted in asymptomatic, full disease control.
Prof Louis emphasised that good communication between physicians and patients results in the development of goals that are both relevant and meaningful to patients. Patient-reported outcomes (PRO) are increasingly included in clinical trials and required by regulatory agencies. Prof Louis described how tools such as the IBD Disk, which was developed in partnership with patients, can highlight issues that impact the patient’s life and therefore aid in optimal communication between physicians and patients.
Symposium Introduction
Professor Remo Panaccione
It is well established that CD is a progressive disease; however, the progressive nature of UC is less widely accepted. The objective of the symposium was to increase awareness of the progressive nature of UC and the importance of treating early, setting treatment goals, and using enhanced clinical monitoring. Additionally, the symposium aimed to highlight the importance of the patient’s perspective beyond their symptoms in order to improve communication with patients on the impact of IBD and its treatment on the broader aspects of quality of life.
Assessing the Course: Understanding Progression in Inflammatory Bowel Disease
Professor Laurent Peyrin-Biroulet
For almost a century, it has been established that IBD is associated with permanent damage to the bowel. In the early 1930s, Burril B. Crohn first described2 the strictures, multiple fistula, disease progression, and bowel damage in regional ileitis, now known as CD, but it took decades for the progressive nature of CD to be fully understood. Prof Peyrin-Biroulet outlined the findings of studies that demontrated the progressive nature of CD. A population-based cohort study3 in 2010 found 18.6% of CD patients experienced penetrating or stricturing complications within 90 days of diagnosis. The cumulative risk of developing either complication was 33.7% at 5 years and 50.8% 20 years after diagnosis. More recent research suggests that the percentage of patients with bowel damage at diagnosis could be even higher. Using cross-sectional imaging, a joint French–Italian group found that 39.4% of patients had bowel damage at diagnosis;4 complications included fistulas, strictures, and abscesses. Bowel damage at diagnosis was associated with a worse prognosis than non-stricturing and non-penetrating disease.4 After a median follow-up of 4.9 years, patients with complicated CD (i.e., bowel damage at diagnosis [n=56]) were significantly more likely to have had intestinal surgery (hazard ratio [HR]: 3.21; p<0.001) and CD-related hospitalisation (HR: 1.88; p<0.002) than those with early-diagnosed CD and no damage (n=86).4 According to Prof Peyrin-Biroulet, such data highlight how one goal of early diagnosis and disease control is to prevent surgery in the long-term.
Drawing on his personal experience, Prof Peyrin-Biroulet suggested that patients can be disappointed by surgical outcomes. Progression in IBD may start with stricture followed by fistula or abscess and the need for surgery. However, a few weeks following surgery, another stricture may occur, and the process may begin again. Prof Peyrin-Biroulet suggested there is a possible window of opportunity early in the course of the disease when progression may be prevented and, thus, early diagnosis coupled with early intervention could result in better disease control.5
The primary benefit of earlier diagnosis and intervention is that anti-TNF therapy may be more effective when used early in the course of CD progression.6 A pooled analysis6 of patients taking adalimumab found that remission rates (measured as Crohn’s Disease Activity Index [CDAI] <150 or Harvey–Bradshaw index <5) were significantly higher in patients with a disease course of <2 years compared with patients who had had CD for >5 years before initiating treatment. Similar results were found in the exploratory analysis of the EXTEND study.7 Of the patients receiving continuous adalimumab (40 mg every other week [EOW]), 33% of patients with early CD (≤2 years) were in deep remission (absence of mucosal ulceration and CDAI <150) at Week 52. Only 16% of patients with a disease duration >5 years experienced deep remission. Prof Peyrin-Biroulet noted that if anti-TNF therapy is introduced soon after diagnosis, the probability of achieving full remission, as assessed by both CDAI and mucosal healing data, is greater.
The key concept in CD management for the next decade will be the importance of the window of opportunity early in disease course when treatment with disease-modifying agents can lead to early and sustained deep remission. As outlined in Figure 1,9 the goal of treatment is to achieve deep remission, including biological and clinical remission and complete mucosal healing, at every evaluation. Data from the CALM study1 suggest that this approach may prevent, or at least impede, disease progression so that CD patients have decreased disability, reduced bowel damage, and are less likely to require surgery.
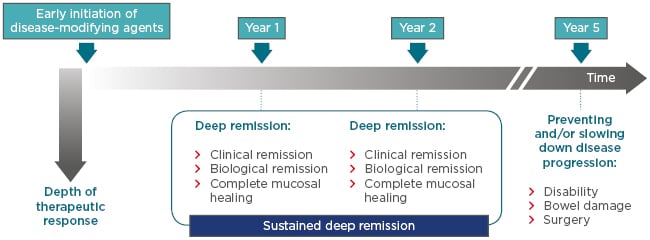
Figure 1: Targeting early Crohn’s disease and achieving sustained deep remission: The best way to change disease course?
The aim of initiating treatment early with disease-modifying agents is to achieve and sustain deep remission; it is not enough to achieve deep remission once. The aim of this treatment approach is to improve disease outcomes in the long-term, as result of slowing down or preventing disease progression.
Adapted from Peyrin-Biroulet et al.8 and Bouguen et al.9
The same approach needs to be adopted in UC. Patients with UC are often undertreated because of perceptions that colectomy is a cure, that UC disease burden is less than the burden associated with CD, and that UC is not a progressive disease. However, these are misperceptions, according to Prof Peyrin-Biroulet. While colectomy is an option in UC and is sometimes necessary or a good option when disease complications, such as strictures, malignancy, or dysplasia occur, colectomy is not always a cure. In the postoperative short term (≤30 days), 21% of patients with UC will develop some complication.10 In the long-term (>30 days), 39% of patients have complications, such as pouchitis (29%), faecal incontinence (21%), small bowel obstruction (17%), fistula (6%), and even mortality (1% in the short term, 0.2% in the long term).10
In terms of disease burden and overall disability, UC and CD have been shown to have a similar impact on patients.11 A study using a quality of life questionnaire assessing fatigue and work productivity among other factors found that the score on the IBD-disability index was 33.9±19.5 for patients with CD (n=150) and 39.2±23.1 for those with UC (n=50).11
With regard to the progressive nature of UC, the extent of colorectal inflammation changes over time and structural changes, such as strictures, pseudopolyposis, and bridging fibrosis, are associated with UC.12 Prof Peyrin-Brioulet also noted that functional abnormalities (decreased contractility and motility, impaired colonic permeability) and anorectal dysfunction (‘lead pipe’ colon, rectal narrowing, and widening of presacral space12) will damage patients’ quality of life.
Furthermore, colonic strictures should raise concerns about the risk of cancer. A nationwide study from GETAID included patients without preoperative evidence of dysplasia or cancer who underwent surgery for colonic strictures. Of 12,013 patients who underwent surgery between 1992 and 2014, 248 patients with CD and 39 patients with UC had low or high-grade dysplasia or cancer.13 Prof Peyrin-Biroulet highlighted that dysplasia and cancer are associated with undertreated disease.
In conclusion, both UC and CD are progressive diseases. The disease burden depends on many factors but is broadly similar in UC and CD. Early intervention and disease control are necessary also in UC to improve short and long term outcomes and to avoid disability and colectomy.
Navigating Outcomes: Optimising Treatment of Inflammatory Bowel Disease
Professor Jean-Frédéric Colombel
The three pillars of optimal care in IBD are early intervention, T2T, and TC,14 all of which are built on a foundation of communication with the patient and hence, require patient empowerment.
A T2T approach involves predefining a treatment target in consultation with the patient, continuously monitoring disease activity, and modifying treatment until the target is reached. The aim is not only to control symptoms, but also to block disease progression in order to avoid bowel damages and disability. In 2015, it was proposed in the STRIDE guidelines8 that the target in CD should be a composite endpoint of clinical and endoscopic remission, with clinical remission defined as resolution of abdominal pain and normalisation of bowel habit that should be assessed every 3 months during active disease. Patients’ individual goals and specific challenges, such as perianal disease, should also be taken into account. Endoscopic remission was defined as resolution of ulceration, assessed by endoscopy 6–9 months after initiation of therapy. When the disease is mainly located in the small bowel, cross-sectional imaging should be used. In 2015, biomarkers such as C-reactive protein (CRP) and faecal calprotectin were not considered targets but, rather, adjunctive measures of inflammation used to achieve TC. Histologic remission was not considered a target in 2015.
In UC, the same concept of a composite target was proposed.8 Clinical remission was defined as resolution of rectal bleeding and normalisation of bowel habit, assessed every 3 months during active disease. Patients’ individual goals including mood disorders, fatigue, and work productivity should be included in the target. Endoscopic remission was defined as the resolution of friability and ulceration, assessed via flexible sigmoidoscopy or colonoscopy (Mayo score 0–1) within 3–6 months of initiation of therapy. Again, biomarkers such as CRP and faecal calprotectin are adjunctive measures of inflammation, not targets, for monitoring UC and histologic remission was not considered a target in 2015 due to lack of evidence of its clinical utility; however, Prof Colombel noted this might evolve.
The final pillar is TC. In order to reach the target, the patients’ symptoms and biomarkers need to be monitored regularly. The two main biomarkers used currently as part of this approach are CRP and faecal calprotectin. The CALM study1 was an open-label, multicentre, Phase III study in Europe and Canada, designed to compare two treatment algorithms: a conventional management approach and TC treatment algorithm in newly diagnosed CD patients. Patients (n=244) received up to 8 weeks prednisolone before randomisation to conventional management or TC. In the conventional management arm, treatment escalation was based on symptoms or steroid use; in the TC arm, treatment escalation was based not only on symptoms or steroid use but also on CRP or faecal calprotectin levels. Assessments took place every 12 weeks. In the TC arm, if a patient was in clinical remission but biomarker levels were raised, treatment was escalated. The escalation sequence was no treatment, adalimumab EOW, adalimumab weekly, and finally adalimumab weekly plus azathioprine. There was an option for rescue if treatment escalation was needed before the next assessment. Treatment could also be de-escalated.
The results from the CALM study show that a higher proportion of patients in the TC arm achieved the primary endpoint of mucosal healing (CDEIS <4) and no deep ulcerations at Week 48 (56/122; 46%) compared with the clinical management group (37/122; 30%). At the end of the study, all secondary endpoints were achieved by more patients in the TC than in the conventional management arm. Deep remission, for example, a composite for clinical and endoscopic remission, was achieved by 36.9% of those in the TC arm compared with 23.0% in the conventional management arm (p=0.014).1 The CALM study concluded that timely escalation with an anti-TNF therapy on the basis of clinical symptoms combined with biomarkers in patients with early CD resulted in better clinical and endoscopic outcomes than symptom-driven decisions alone.
Escalation of therapy based on biomarker levels meant that, overall, patients in the TC arm received earlier and more intensive treatment. Prior to randomisation, the reasons for escalation were similarly represented and included symptoms (CDAI, prednisone use), as well as biomarker levels (CRP and faecal calprotectin). As the study progressed, escalation became primarily driven by biomarker levels.
De-escalation of treatment once targets are reached remains a topic of interest, but Prof Colombel urged caution when considering de-escalation because of the lack of evidence to support it. Further analysis of the CALM data15 found that, in the small numbers of patients who de-escalated treatment, 61% of those in TC arm (n=23) and 54% in the clinical management arm (n=13) achieved mucosal healing at Week 48. In the TC arm, 75% (n=8) of patients who de-escalated treatment and then required re-escalation achieved mucosal healing at Week 48.
A key finding in follow-up of the CALM data was that, 1 year after randomisation, there were significantly fewer CD-related hospitalisations in the TC group (13.2 events/100 patient-years; n=122) compared with the clinical management group (n=122; 28.0 events/100 patient-years; n=122; p=0.021).16 A 5-year follow-up of CALM hospitalisation data is expected soon.
Prof Colombel stressed that the concept of TC and monitoring is simple to implement in practice (see Figure 2). Patients are stratified according to their symptoms and objective data, especially from endoscopy. Treatment is initiated and, at 3 months, CRP and calprotectin levels are monitored. At 6–9 months, colonoscopy is performed and if the target (no symptoms, no positive surrogate marker, and no mucosal ulceration) has been achieved, treatment is continued. If not, the treatment is optimised or changed.
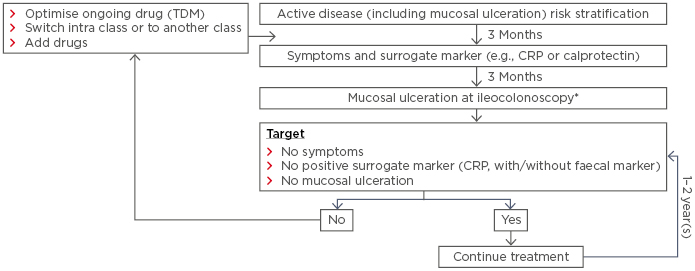
Figure 2: Implementation of treat to target with tight control and monitoring in practice.
*Or resolution of findings of inflammation on cross-sectional imaging in patients who cannot be adequately assessed with ileocolonoscopy.
CRP: C-reactive protein; TDM: therapeutic drug monitoring.
Adapted from Bouguen et al. 9
In conclusion, T2T and TC are complementary approaches that should be tailored to the individual patient. The STRIDE guidelines8 for endoscopic mucosal healing are based on a post hoc analysis and there is currently no prospective study demonstrating that treating to endoscopy is more effective than to symptoms. However, the prospective REACT2 trial17 is ongoing.
Breaking Down Barriers: A Patient Case of Treat to Target
Professor Remo Panaccione
To illustrate how to apply the discussed strategies in clinical practice, Prof Panaccione presented two cases: Case 1 from 2013 and Case 2 from 2018. Both were 22 years old at the time of admission with similar symptoms, including weight loss, abdominal pain, and diarrhoea. Case 1 took 3 years to seek help; Case 2 took 3 months. Consequently, at diagnosis, Case 1 had anaemia, was iron-deficient, and was malnourished; Case 2 was not. Case 1 had to wait 6 months to be referred to a gastroenterologist; Case 2 benefitted from an expedited referral and was seen in 4 weeks. At the initial consultations, examination revealed that both women had a 20 cm deep ulceration in the terminal ileum, 30 cm ileal inflammation, and mild narrowing. Case 2 also had biomarker assessment indicating normal CRP but a faecal calprotectin level of 800 µg/g. In both women, prednisolone was initiated and both experienced symptom improvement at 3-month follow-up. Case 1 underwent no additional monitoring, but Case 2’s biomarkers were assessed again: calprotectin was 450 µg/g, lower than at diagnosis, but too high to be indicative of controlled inflammation. Based on discussion with her physician, despite feeling better, Case 2 initiated treatment with adalimumab (induction dose 160/80 mg; 40 mg EOW).
At 6 months, Case 1 had recurrent symptoms and started on prednisone and azathioprine. Case 2 was asymptomatic, but the calprotectin level remained elevated (390 µg/g) and consequently her adalimumab dose was increased to 80 mg EOW. She remained asymptomatic on this dose thereafter, with fully controlled disease.
At 9 months, Case 1 was admitted to hospital with pain, bloating, and vomiting; she received intravenous corticosteroids, an induction dose of adalimumab, and continued treatment with 40 mg EOW. She continued to have intermittent symptoms, switched to infliximab therapy with no improvement, and underwent a 40 cm ileocaecal resection with primary anastomosis.
Prof Panaccione commented that the management seen in Case 1 is common in practice: delayed diagnosis, a lack of monitoring and no optimisation of treatment, and biologic treatment initiated too late. Disease progression and advanced structural damage were the consequences in Case 1’s case. By contrast, the strategy of T2T and TC used in Case 2, with decisions based on discussion, education, and monitoring, resulted in full disease control. It was noted that physicians must respect the values of patients and their views on the therapeutic journey regarding monitoring and optimising treatment. However, research from CALM,1 among other studies, suggests that the management of IBD will evolve significantly, not because of different therapeutic agents, but due to different implementation strategies.
Looking Beyond Inflammatory Bowel Disease: Effecting Change in Patients’ Lives
Professor Edouard Louis
IBD has an impact on patients’ lives, not only during periods of active disease, but also between flares. The IMPACT study,18 conducted by the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) included 4,670 patients with IBD. The study found that, even between flares, almost half (49% of CD patients; 47% of UC patients) responded that their life was somewhat or significantly affected by the disease during the course of everyday activities.
The IMPACT study18 also found that the majority of patients would like better communication with their gastroenterologist. Most (64%) felt that the gastroenterologist should ask more probing questions (sometimes or more frequently), while 31% of respondents were satisfied with their consultations. Similarly, 54% of patients reported that they had no opportunity to tell their gastroenterologist something potentially important (sometimes or more frequently) compared with 41% who were satisfied.
Prof Louis felt this finding may explain the discrepancy between physician and patient assessment of disease, with physicians reporting full control of disease more frequently than their patients. For instance, a web-based questionnaire, completed by adult UC patients and their physician in Europe and Canada, found that 43% of physicians (n=475) believed their patients’ symptoms to be completely or mostly under control. By contrast, only 26% of patients (n=775) reported this to be the case.19
As a result of such findings, both the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) have implemented new criteria for drug development in IBD20,21 that include both mucosal healing and validated PRO. The EMA guidance states that coprimary endpoints in UC are endoscopic Mayo score of 0–1 and no reports of PRO, including bleeding. Likewise, in CD, mucosal healing is assessed by a score (e.g., Crohn’s Disease Endoscopic Index of Severity [CDEIS]=0) and the PRO include number of stools and abdominal pain.
Increased efforts are being made to report PRO. The PYRAMID registry22 includes patients with CD treated with adalimumab and followed for up to 6 years. Scores from the health-related Short IBD questionnaire completed by these patients suggest a clinically meaningful improvement of ≥9 points from baseline at 1 year and maintained over 6 years. The work productivity and activity impairment index (WPAI) is used to assess four domains: absenteeism, presenteeism (under-performance at work), overall work impairment, and activity impairment. Patients included in the PYRAMID registry22 reported clinically meaningful improvements in WPAI across all domains at almost all time points (defined as an improvement of ≥7 points from baseline at <2 years, 2–<5 years, 5–<10 years, and ≥10 years).
Disease duration prior to initiation of treatment was shown to be important. Numerically greater improvements in overall work impairment, for example, were seen in patients with CD duration <5 years compared to ≥5 years. The impact of adalimumab is clinically meaningful in both groups but is less pronounced when treatment is introduced later in the course of disease. Prof Louis said there is disease progression at both the tissue and the psychosocial level.
A variety of PRO have been used in clinical trials, but, according to Prof Louis, many have been developed without the participation of patients.23 Therefore, they do not always tackle the questions that are most relevant to the patient. He advocated the use of communication tools such as the IBD Disk, which was based on the validated disability index.11 Patient focus groups selected relevant issues that were then agreed upon by an expert consensus group.24 The IBD Disk includes 10 items: abdominal pain, regulating defecation, interpersonal interactions, education and work, sleep, energy, emotions, body image, sexual functions, and joint pain. Patients score each item (0–10) on a coloured disc, which results in a highly visual tool for assessing IBD-associated disability. Frequent use of the tool allows the impact of the disability to be followed over time, disease management goals to be set for the short and long term, and treatment efficacy to be monitored. It may also encourage adherence to medication by demonstrating to patients that the treatment is impacting issues that are important to them personally. This tool provides a comprehensive assessment of quality of life and alongside this also highlights specific problems and may therefore facilitate discussions between the patient and the physician that might not have occurred without its use. Physicians using the IBD Disk or other tools must be prepared to address issues raised as a result of this assessment. A collective approach involving other professionals, such as psychologists, dieticians, social workers, and nurses, can assist patients in coping with their disease in daily life.
In conclusion, Prof Louis stressed the importance of discussions with patients on the burden of disease beyond their clinical symptoms, including quality of life, daily living, and work productivity. The IBD Disk is a good example of a tool developed in partnership with patients that highlights broad and/or specific disease-related issues that impact patients’ daily life.
Panel Discussion Points
- The STRIDE guidelines8 include a target of complete mucosal healing. Indirect evidence suggests that a target of histological healing may improve outcomes further, but there is insufficient evidence to implement this in clinical practice today. Treating to more stringent targets will lead to more failure of therapy and potentially lower adherence.
- The suggested threshold at which treatment is escalated is changing frequently as new evidence becomes available, but regular monitoring is undisputed. Monitoring and treatment optimisation may be cost-effective if savings from decreased hospitalisation, physician consultations, and work productivity are taken into account.
- Physicians are urged to not undertreat, especially in cases of proctitis. They may be reluctant to initiate biologic therapy for 5–10 cm of disease activity, but loss of rectal function leads to distressing symptoms such as faecal urgency and incontinence. Early control of inflammation in UC is essential to maintain function.
- Disease progression assessment should include psychosocial damage and motivate physicians to initiate treatment early in disease. Loss of professional and/or social life may be irreversible and may be as important to patients as tissue damage.
- TC is feasible in clinical practice when patients are motivated; non-adherence may occur if they do not understand the rationale. Targets which integrate quality of life factors may increase patients’ motivation.
- There is currently a lack of evidence on dose de-escalation but the topic should be discussed with patients in order to decrease the possibility of patients stopping treatment without medical supervision.
Conclusion
Prof Panaccione closed the symposium by stressing that both CD and UC are progressive diseases. Early intervention and personalised risk stratification are part of a T2T strategy in which the target is a composite endpoint of clinical and endoscopic remission, agreed in discussion with the patient. The CALM study demonstrated that timely escalation with an anti-TNF therapy on the basis of clinical symptoms combined with biomarkers in patients with early CD resulted in better clinical and endoscopic outcomes than symptom-driven decisions alone.
A T2T strategy involves TC and prevention of disease progression. Regular disease monitoring through visits, biomarker assessment, and timely endoscopy is essential. Current evidence suggests that this approach can change the course of IBD, but more data are needed to confirm the long-term benefits.
Additionally, patient factors beyond clinical symptoms must be considered. PRO tools and communication strategies can enhance patient engagement in shared decision-making and help physicians support patients in achieving both clinical goals and those involved in succeeding in their daily life.
Prof Panaccione noted he believes that the course of IBD can be changed and commented that early intervention, T2T, and TC are the pillars for supporting change, all of which are based on a foundation of good and open communication with the patient.








